Battery Characterization in Today’s World
Batteries have powered life around us for years, from household products to life-saving medical devices, and even our favorite toys as children. Today there are more applications for batteries than ever before, influencing a surge in research to explore how they can be used to sustainably power our future.
How does a lithium-ion battery work?
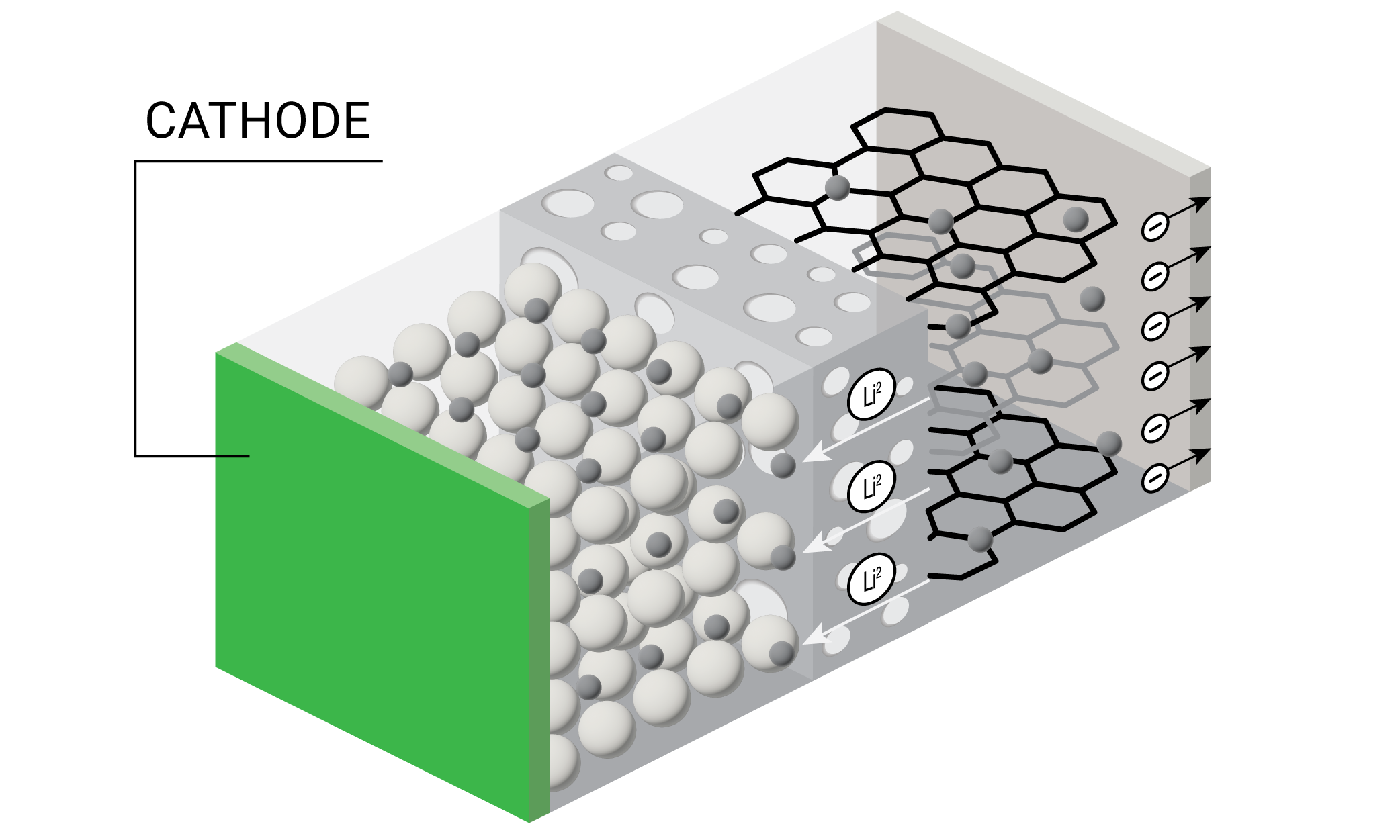
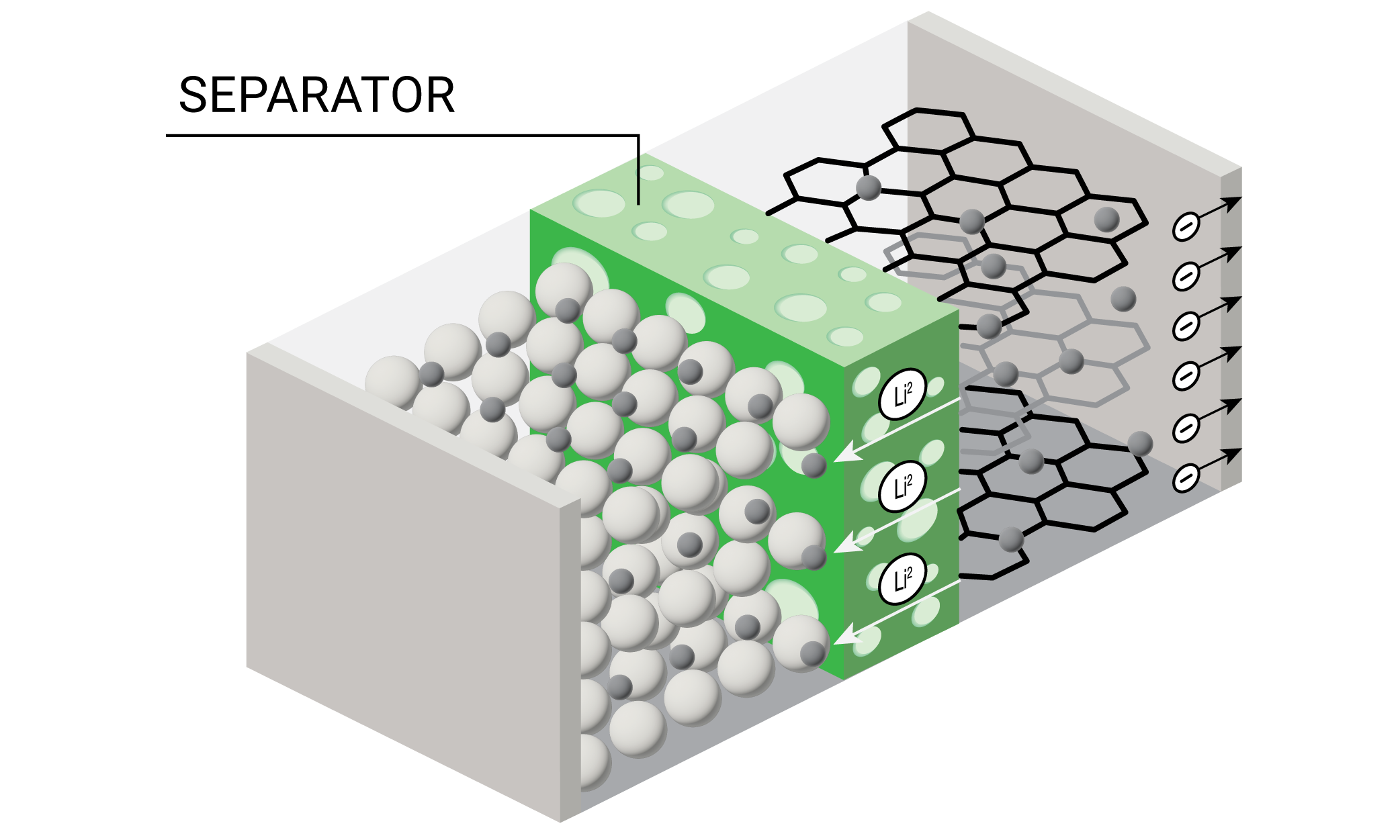
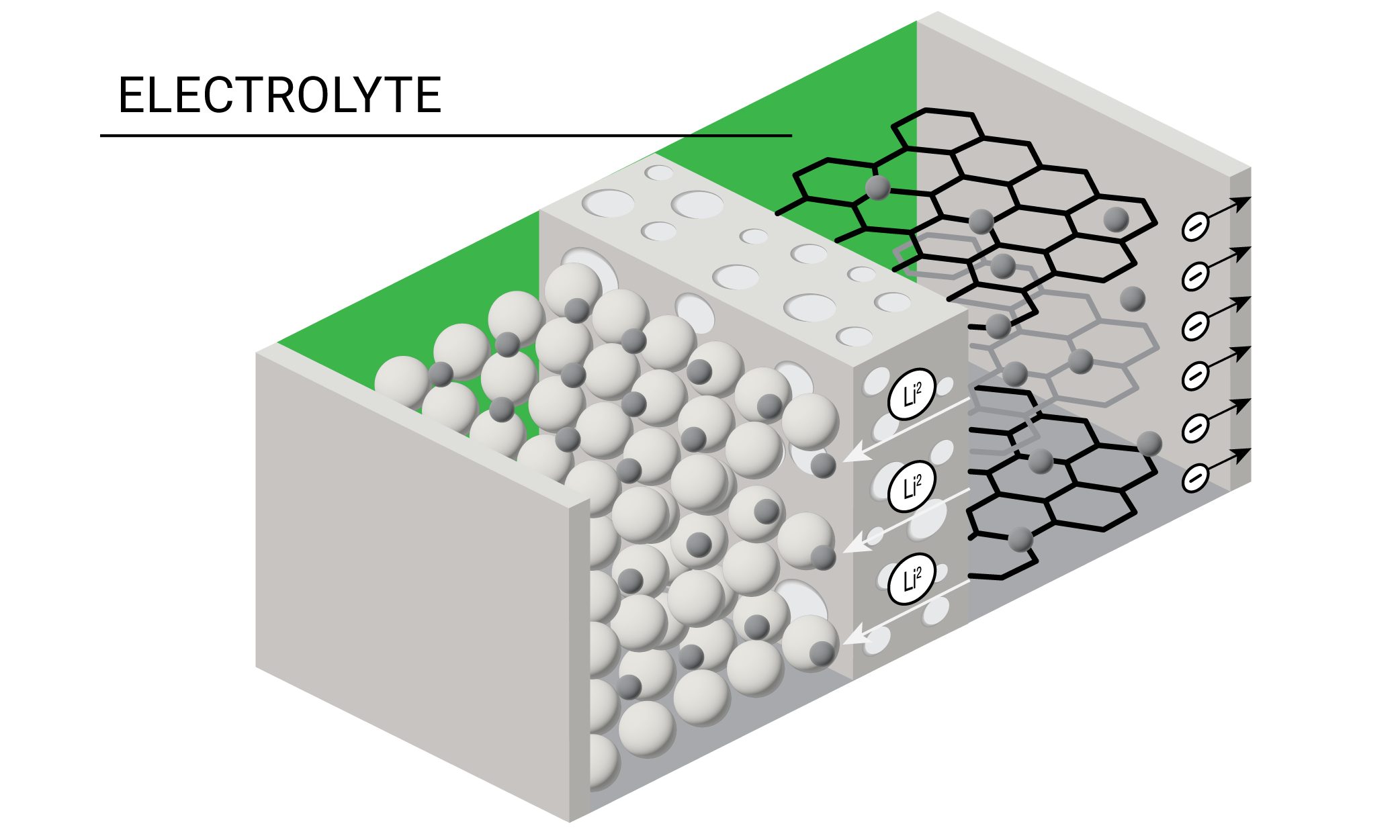
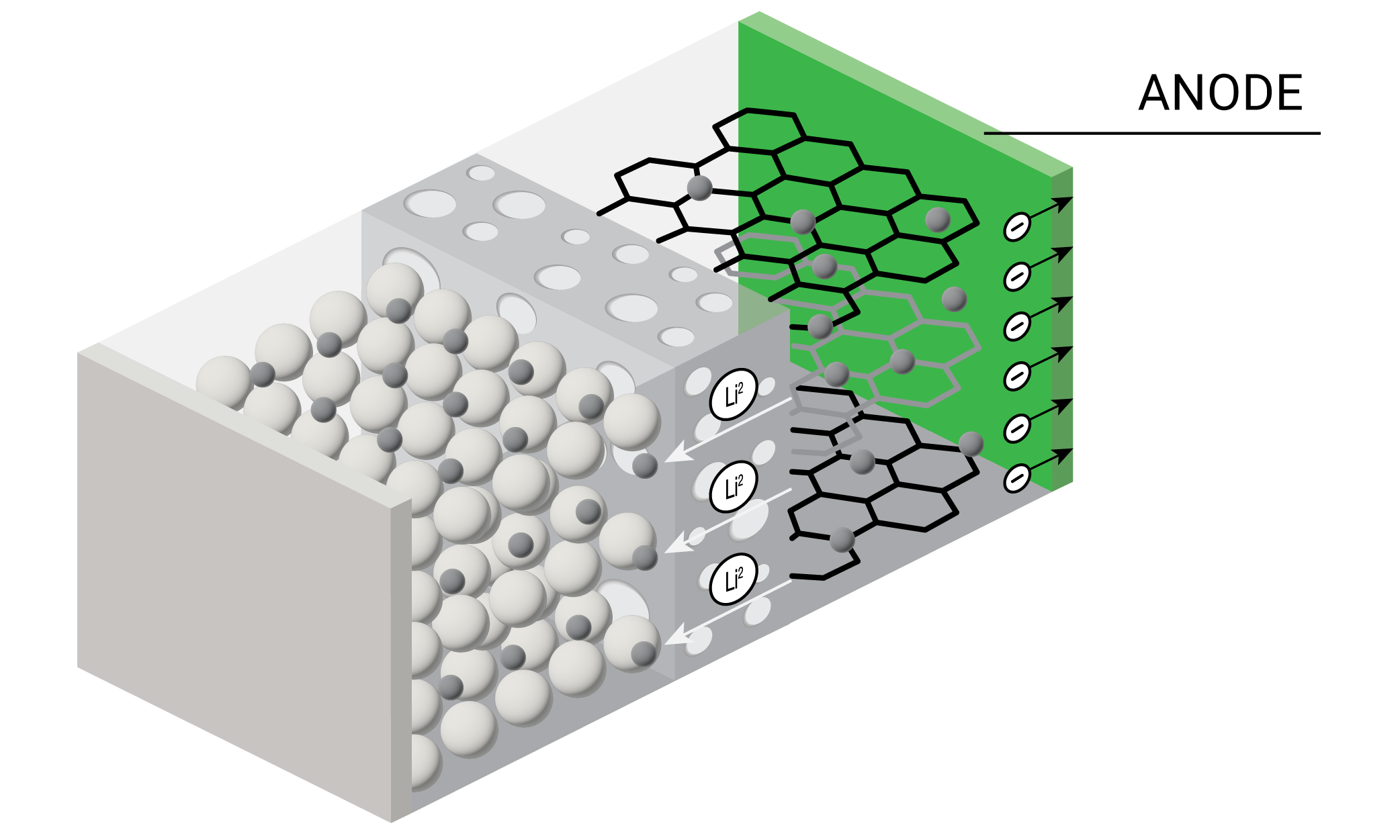
A lithium-ion battery is comprised of four main components – cathode, anode, separator, and electrolyte. In a working battery, lithium ions flow from the anode to the cathode during discharge. The lithium-ions flow in the reverse direction during recharging. Each individual battery cell outputs only a limited amount of energy and is often combined with other cells to form battery packs. Battery packs can in turn be combined to form battery modules for energy storage applications that require higher amounts of energy output such as electric vehicles and grid storage. The materials comprising the cathode, anode, separator, and electrolyte together help define a battery’s six primary performance characteristics – run time, safety, cycle life, power, energy density, and costs.
Learn more about how the Rheo-Impedance Spectroscopy can help you optimize your battery electrode slurries.
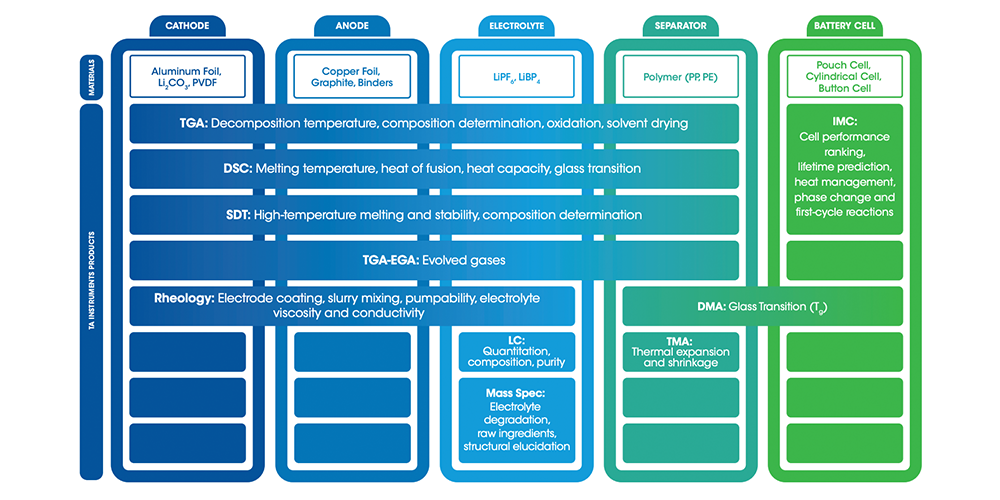
Making a Battery: How to Choose the Right Materials
A key aspect of selecting the best materials for each part of the battery to optimize the six primary performance characteristics depends on advanced analytical characterization. One of the most critical parameters in material selection is thermal tolerance because the materials comprising a working battery must work within a temperature range of -20 °C to 60 °C. The analytical technique known as thermal analysis is ideal for testing thermal tolerances and stability of battery materials. With thermal analysis, it’s possible to obtain such thermal parameters as the decomposition temperature, chemical composition, degree of oxidation, solvent composition, melting temperature, glass transition, and thermal stability.
Battery Components
Learn more about the materials and processes that go into creating each part of a battery.
When thermal analysis is combined with mechanical testing, it’s possible to understand dimensional stability of a polymer’s length and shape, e.g., the polymer separator. This thermal and dimensional insight can help prevent separator failure and ensure battery safety.
Finally, when it comes to assembling the materials together during manufacturing, it often requires working with slurries of solid particles, binders, and solvents. At this stage, rheology provides critical insights into battery slurries at each manufacturing stage including storage, mixing, coating, and drying. A rheological profile measurement can help ensure a uniform, defect-free coating that leads to production of consistent, high-quality electrodes with high batch-to-batch repeatability and low scrap rates.
Whether your goal is to create a higher performing battery within a smaller footprint or develop a brand-new battery using more sustainable materials, knowing the thermal, rheological, calorimetric, and mechanical properties of the materials in your main battery components is key to your success. Waters-TA Instruments provides leading battery researchers with the advanced analytical characterization tools needed to develop higher performing and safer battery technology. Contact us today to start designing your personal workflow for success.
Resources
Webinars
Blog
Application Notes
- Determination of Parasitic Power in Lithium-ion Batteries using the Battery Cycler Microcalorimeter Solution
- Safety Evaluation of Lithium-ion Battery Cathode and Anode Materials Using Differential Scanning Calorimetry
- Rheo-Impedance Measurements in Li-Ion Battery Research: Additive Effect of Carbon Nanotubes in LiFePO4 Cathode
- Rheological Evaluation of Battery Slurries with Different Graphite Particle Size and Shape
- View all application notes