A cell is the smallest, packaged form a battery can take. Lithium-ion battery cells come in four formats: cylindrical, prismatic, pouch, and coin cells. The former three are used in products while a coin cell is typically only used for research purposes. Testing battery cells is an important step in optimizing battery design and components before incorporating cells into larger modules and battery packs. To understand the impact of each component to the cell performance see our analytical testing solutions for the cathode, anode, and separator.
Battery scientists need to determine cells’ efficiency as well as degradation during cycling. When isothermal microcalorimetry (IMC) is paired with a cycler or potentiostat, critical insights on lifetime predictions, cell performance rankings, and heat management evaluation can be made.
TA Instruments Battery Cycler Microcalorimeter Solution offers the highest throughput, integrated, and sensitive solution to get the most out of your battery testing, drastically reducing experimental time from months to weeks for data that must be replicated numerous times.

Instrument & Test Parameters
Battery Cycler Microcalorimeter Solution
Cell performance ranking
Lifetime prediction
Parasitic reaction detection
Heat management
Phase change and first cycle reactions
Application Example
In Operando Calorimetric Testing of Battery Whole Cells
The electrochemical processes that take place in batteries, whether under load or charging conditions give rise to heat exchange with the surroundings. The work performed as charged species flow internally in a cell gives rise to heat generation as well as the redox processes at the anode and cathode and various parasitic side reactions that are responsible for limiting the service life of a battery. Isothermal Microcalorimetry (IMC) is a non-specific and non-destructive technique for measuring the smallest reactions in a material during a physicochemical process. This is done by measuring the heat flow from the sample at a constant temperature. In battery research, isothermal calorimetry of li-ion battery cells covers three main areas of interest:
- The first is the thermal output of a cell from the point of view of heat management.
- The second is the understanding of structural evolutions in active materials as evidenced by entropy changes.
- The third is the isolation of heat from parasitic reaction to rank the performance of cells. Evaluations of pouch, coin, pacemaker, cell phone, and cylindrical batteries can be conducted under passive storage conditions or in tandem with a battery cycler.
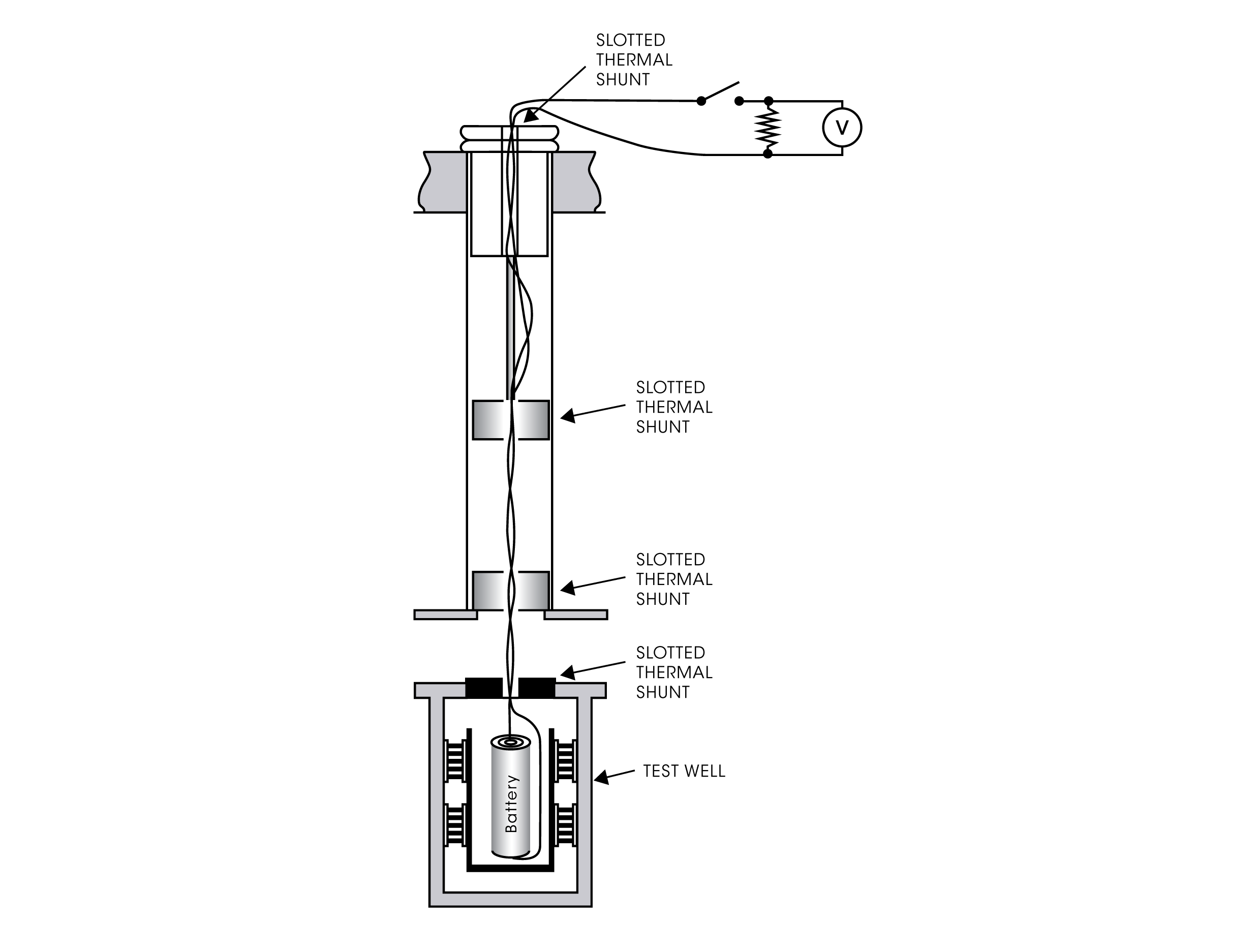
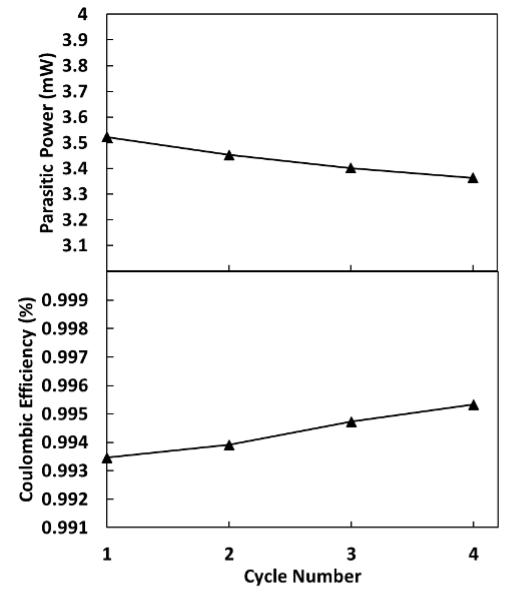
The Battery Cycler Microcalorimeter Solution combines sensitive isothermal microcalorimetry with electrochemical analysis in one integrated hardware and software solution. Using this methodology, valuable insights on cell behavior can be determined. Figure 1 demonstrates the easy set-up of the battery lifter that is used in the microcalorimeter. The chart in Figure 2 depicts data analyzed using TAM Assistant. It shows the cell’s average parasitic power and the Coulombic efficiency across four cycles. The Coulombic efficiency is a measure of the electrochemical efficiency; conversely, the parasitic power is a measure of the cell’s inefficiency, including both chemical and electrochemical side reactions.
Application Notes