Thermal Analysis in Pharmaceutical Research, Development, and Quality Control
Monika Schennen | Julienne Regele | Calliste Scholl | Morgan Ulrich
April 18, 2024
As a central pillar of modern society, the pharmaceutical industry bears the load of billions of lives around the world. In 2022, the global revenue of the pharmaceutical industry approximated $1.5 trillion, a figure reflected in two decades of significant growth.1
Pharmaceutical development is advanced by the continued search for new active pharmaceutical ingredients (APIs), including analogs, phytopharmaceuticals, and biopharmaceuticals, which have the potential to yield life-changing drugs. This enterprise is concomitant with an increasing trend toward designing new dosage forms and drug combinations, improving manufacturing processes, and exploring new indications for existing drugs.

What is Thermal Analysis?
Addressing the needs of the pharmaceutical industry, specifically the research, development and analysis of drugs, requires a sophisticated suite of techniques for fast and effective characterization and quality control. Thermal analysis is a family of techniques that measures the change of specific properties of materials as a function of temperature to elucidate their physical and chemical characteristics.2,3 While thermal analysis comprises numerous methods, this article will focus on three key examples: differential scanning calorimetry, thermogravimetric analysis, and sorption analysis.
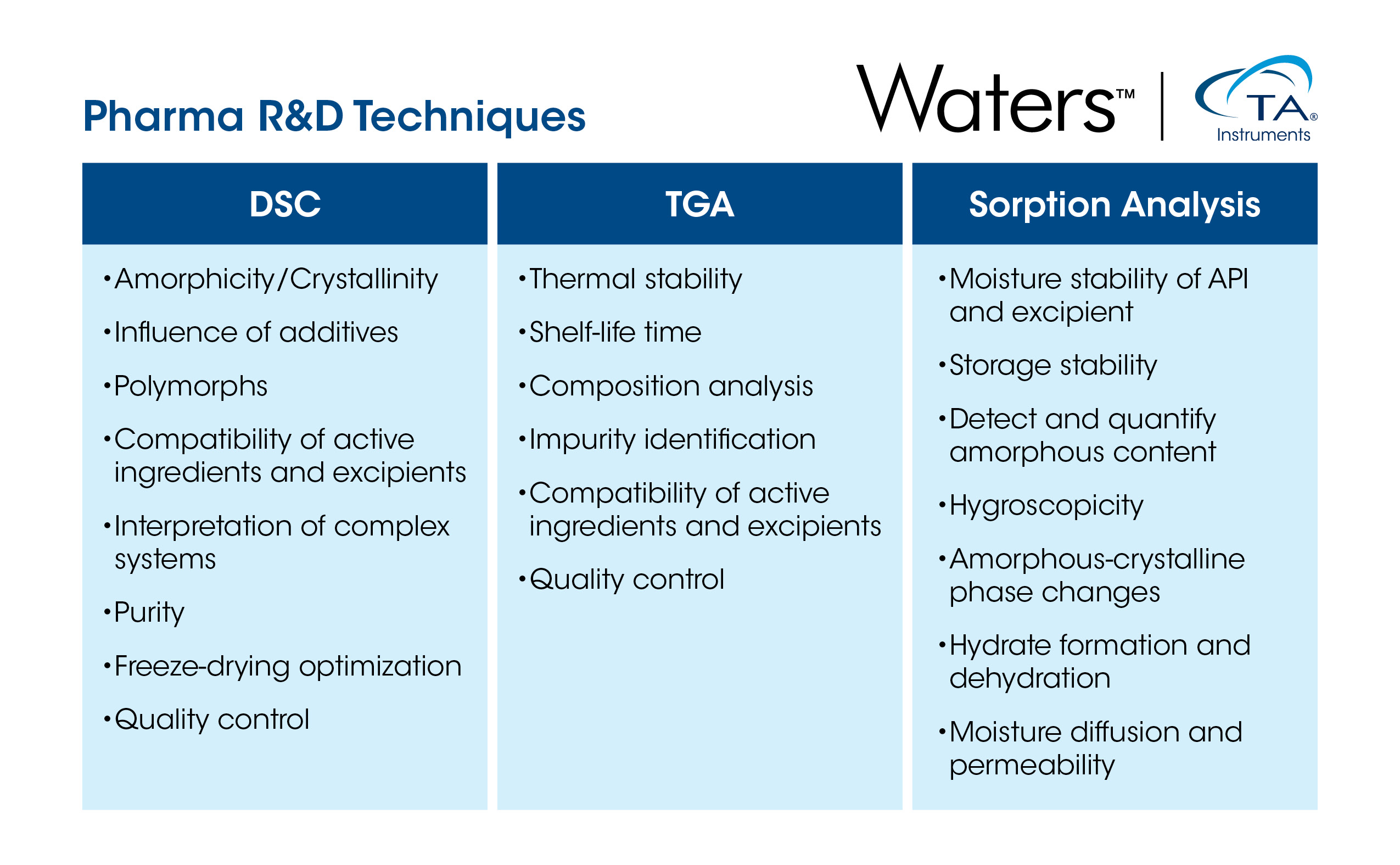
Thermogravimetric Analysis: Capturing the Interactions Between Temperature and Material Weight
Thermogravimetric analysis (TGA) is a valuable analytical technique employed in pharmaceutical R&D to assess the thermal stability and decomposition behavior of materials. TGA involves subjecting a sample to controlled temperature increases under a controlled atmosphere while continuously monitoring its weight.4
Material stability is of paramount importance in pharmaceuticals, as it directly affects product safety, efficacy, and shelf-life. Understanding how a pharmaceutical substance reacts to temperature variations and identifying potential degradation pathways is critical for ensuring the stability of the final product.
TGA offers several benefits to pharmaceutical research and development, including:
- Identifying optimal storage conditions and packaging materials to prevent premature drug degradation through precise degradation measurements
- Determining the moisture content of products, which directly affects chemical stability, API crystal structure, dissolution rate, and compaction.5
- Ensuring the development of stable and effective drug formulations by selecting thermally compatible excipients and formulation components.
Providing quality and regulatory compliance when coupled with mass spectrometry Fourier-transform infrared spectroscopy (FTIR). Together, these techniques can detect and quantify volatiles in pharmaceutical samples.
Differential Scanning Calorimetry: Capturing Material Temperature Changes in Response to Environmental Temperature
Differential scanning calorimetry (DSC) is a powerful analytical technique widely used in the pharmaceutical industry to characterize APIs and excipients. DSC measures the heat flow associated with temperature changes in a sample during heating, cooling, or remaining isothermal, offering insights into phase/structural changes, chemical reactions, and physical interactions.4
During drug research DSC is critical in identifying polymorphs, or different forms of the same chemical compound. Different polymorphs of a drug can exhibit distinct physicochemical properties, such as solubility, dissolution rate, and bioavailability. By subjecting a sample to varying temperatures, DSC can reveal different polymorphs and aid in the selection of the most desirable form, for example, optimizing for enhanced bioavailability and manufacturability.4
Additionally, DSC provides critical insights into the glass transition temperature (Tg). This is the temperature at which an amorphous material undergoes a reversible transition from a ductile state to a hard/brittle state or vice versa. The size of the glass transition step is linearly proportional to the amount of amorphous structure in the sample, making it a valuable tool for understanding amorphous content.4
Understanding the amorphous content of pharmaceutical solids is advantageous because it can inform decisions to improve the oral bioavailability of poorly water-soluble drugs. Amorphous pharmaceuticals are more soluble and exhibit higher dissolution rates compared to their crystalline counterparts. By monitoring and maintaining amorphous content, DSC can help ensure the stability of these valuable properties.4
Another benefit of DSC is its ability to examine material compatibility. In pharmaceutical formulation, it is crucial to ensure that APIs are compatible with excipients, such as bulking agents and lubricants. DSC can detect incompatibilities by revealing shifts or deviations in thermal behavior, helping formulators make informed decisions to avoid potential issues during drug development. These issues could take the shape of unforeseen chemical interactions between excipients and the API, mismatching of physical properties like solubility and dissolution rates, and impairment of the API’s bioavailability.4
Finally, knowledge of amorphous content aids in optimizing the lyophilization process. Lyophilization, or freeze-drying, is a crucial step in preserving and transporting pharmaceutical products and ensures the stability and efficacy of the final product. For more information on lyophilization, read our blog, How to Optimize Lyophilization with Thermal Analysis.
Sorption Analysis: Capturing the Interaction of Materials and Solvents
Sorption analysis (SA) is a fundamental tool in pharmaceutical R&D used to measure the weight change of a material under water vapor as a function of humidity and temperature. Accordingly, SA helps quantify how moisture is absorbed or desorbed by a drug substance or formulation under various environmental conditions.4 This is important because moisture content is a critical factor that affects the stability of solid dosage forms and can adversely impact the efficacy and shelf life of pharmaceutical products by promoting degradation, such as through the hydrolytic degradation of drugs with certain functional groups.5
SA can also reveal how water sorption can decrease the glass transition temperature of the amorphous phase in a material. This reduction in Tg can soften the amorphous phase and potentially initiate unwanted crystallization, adversely affecting the product’s properties and stability.
Altogether, understanding the moisture sorption behavior of pharmaceutical products ensures that they maintain their desired characteristics, stability, and effectiveness throughout their shelf life. Furthermore, it allows for optimizing formulation strategies, packaging design, and storage conditions.
Thermal Analysis: an Essential Method in the Pharmaceutical Industry
Thermal analysis, comprising techniques such as DSC, TGA, and SA, plays an indispensable role in pharmaceutical research, development, and quality control. These methods are part of a comprehensive toolkit for characterizing pharmaceutical materials and ensuring the safety, efficacy, and stability of drug products. While they can be used in isolation, their use in combination can yield deeper insights into pharmaceutical materials. For example, combining DSC and TGA allows for the detailed examination of decomposition behavior and apparent melting.
TA Instruments’ DSC, TGA, and SA technology provide the most accurate and reliable information about pharmaceutical materials, facilitating the development of safe, effective, and stable drug products for patients worldwide.
Contact TA Instruments’ experts today to learn more about how our technology can revolutionize your research and development process, ensuring the highest standards of quality and efficiency.
References and Further Reading:
- Mikulic, M. (2023). Global pharmaceutical industry – statistics & facts. Statista. Available at: https://www.statista.com/topics/1764/global-pharmaceutical-industry/#topicOverview (Accessed on 01 October 2023).
- Stodghill, SP. (2010). Thermal Analysis – A Review of Techniques and Applications in the Pharmaceutical Sciences. American Pharmaceutical Review. Available at: https://www.americanpharmaceuticalreview.com/Featured-Articles/36776-Thermal-Analysis-A-Review-of-Techniques-and-Applications-in-the-Pharmaceutical-Sciences/
- Duncan, QM., et al. (2006). Thermal Analysis of Pharmaceuticals. CRC Press: Taylor & Francis Group.
- TA Instruments. [Webinar] Thermal Analysis in the Pharmaceutical Industry: Use of TGA, SA, and DSC in Research, Development, and Quality Control. Available at: https://www.tainstruments.com/thermal-analysis-in-the-pharmaceutical-industry-use-of-tga-sa-and-dsc-in-research-development-and-quality-control/ (Accessed on 03 October 2023).
- CEM Corporation. (2021). Moisture Analysis in the Pharmaceutical Industry. AZoM. Available at: https://www.azom.com/article.aspx?ArticleID=18029 (Accessed on 03 October 2023).
Other Resources
- eBook: Pharmaceutical Discovery and Formulation
- Webinar: Characterization of Amorphous Pharmaceuticals by DSC Analysis
- Webinar: Thermal Analysis in the Pharmaceutical Industry: Use of TGA, SA, and DSC in Research, Development, and Quality Control
- App Note: “Apparent Melting”: A New Approach to Characterizing Crystalline Structure in Pharmaceutical Materials
- App Note: Drug – Excipient Incompatibility with Discovery X3
- Contact Us

