Keywords: DSC, Drug-Excipient, Pharmaceutical, Discovery X3
TA442
Introduction
Active pharmaceutical ingredients (APIs) are often processed with other excipients to improve processing, stability and bioavailability. It is important to ensure the excipients are compatible with the APIs and do not affect the chemical or physical properties, and stability of the drug. Thermal analysis techniques are extremely useful tools to study drug-excipient incompatibility. Differential Scanning Calorimetry (DSC), in either standard or modulated mode (MDSC) can be used to detect any drug-excipient interaction that may affect chemical and physical changes in the formulation. [1-3]
In the pharmaceutical industry, rapid screening of drug-excipient interaction is key in the discovery and formulation development of new, potentially lifesaving drugs. The Discovery X3 DSC features a multi-sample cell that can measure highly sensitive heat flow data for up to three samples, (e.g. API, excipients, and mixture of drug-excipients), simultaneously to significantly improve throughput. This application note reports the analysis of drug-excipient incompatibility with the multi-sample Discovery X3 DSC.
Experimental
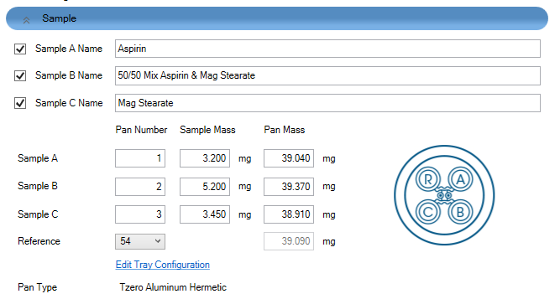
Drug-excipient incompatibility studies were characterized with a TA Instruments Discovery DSC X3 in a single run. Magnesium stearate is a common excipient in pharmaceutical tablet production used as a lubricant. Aspirin is a commonly used API and is known to have interactions with magnesium stearate which induces degradation of Aspirin.[4] Three samples of Aspirin, magnesium stearate, and a 50/50 mixture of Aspirin/magnesium stearate are used to demonstrate this interaction. The three samples were loaded on sample positions (A, B and C).
The experimental setup in TRIOS software is shown in Figure 1. The Discovery X3 DSC contains 4 sensors with one reference (R) and 3 sample positions (A, B and C). The X3 cell layout picture allows users to locate the sensor positions accordingly.
Drug-excipient incompatibility is detected by apparent melting peak with DSC analysis using a heating rate of 1 °C/min from a starting temperature of 10°C to an end temperature of 160 °C with 5 mg of sample in a Tzero hermetic pan. [1-2] A slow heating rate is required to allow more time for the reaction to occur. All 3 samples, Aspirin, magnesium stearate and 50/50 mixture of Aspirin/magnesium stearate, ran in a single test in the Discovery X3 DSC.
Quasi-isothermal MDSC is used to evaluate long-term drug-excipient stability. An isothermal temperature is typically selected at 10 °C below the apparent melting onset transition. [1,3] Quasi-isothermal MDSC at 50 °C was used in this case with +/- 2 °C temperature modulation and 200 sec periods for 24 hr. All 3 samples ran in a single test in the Discovery X3 DSC. The change of reversing heat capacity is an indication of the structure of the sample changed over time.
Results and Discussion
Drug-Excipient Incompatibility in a Single Run
Aspirin has been shown to display apparent melting in DSC experiments as it loses crystalline structure due to decomposition or change of chemical structures. [2] The apparent melting is due to a kinetic process and a change of heating rate will shift the onset temperature. To identify the drug-excipient incompatibility, the apparent melting of API, excipient, and API-excipient mixture are required. With a conventional single stage DSC instrument, three separate runs are required. In the Discovery X3 DSC the 3 samples (API, excipient and API-excipient mixture) all ran at the same time and under the identical temperature profile.
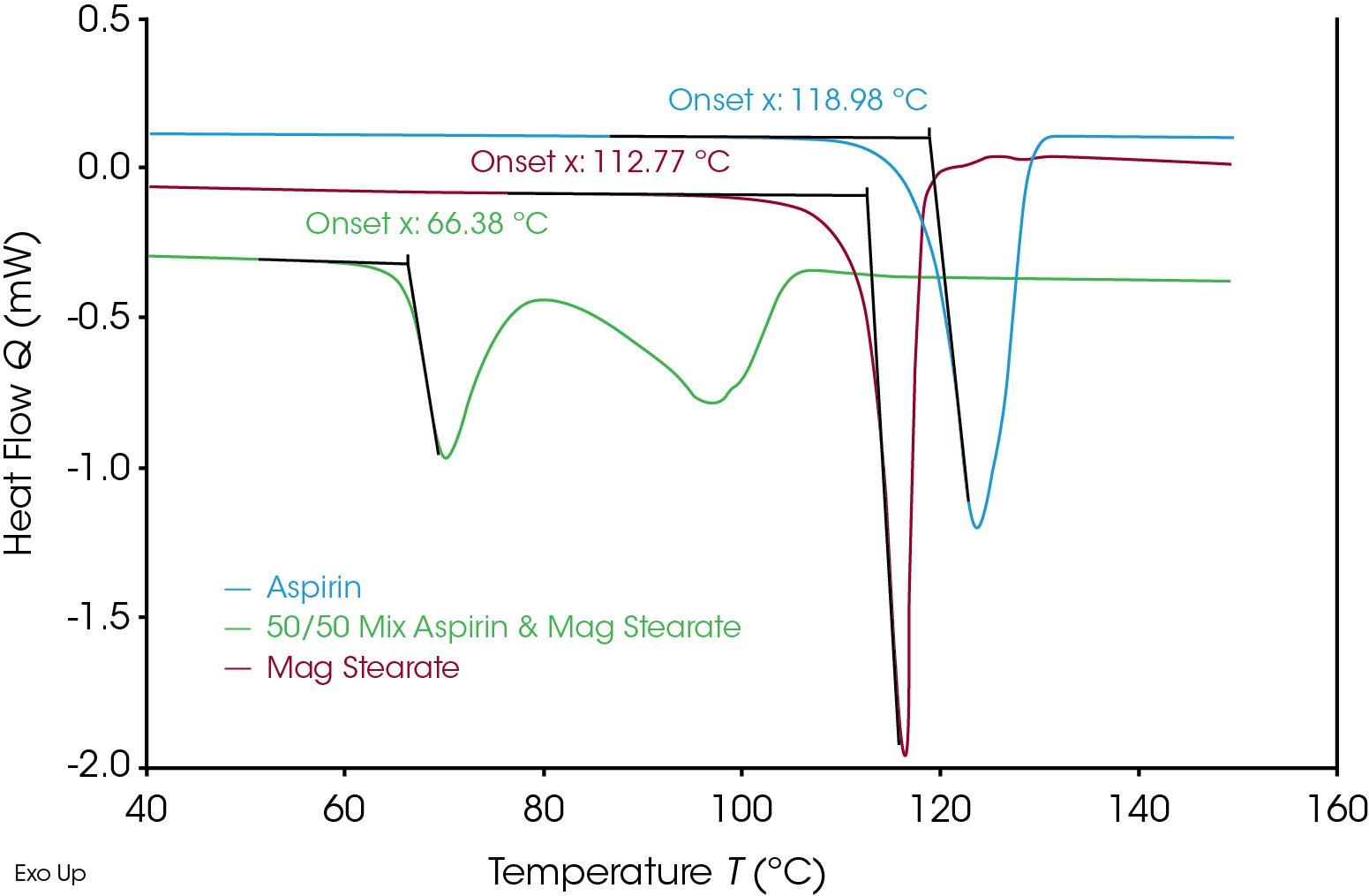
Figure 2 shows the heat flow data from these 3 simultaneous experiments run on the Discovery X3 DSC. Aspirin shows the onset of apparent melting at 118.98 °C and magnesium stearate shows the onset at 112.77 °C. The mixture of the 50/50 Aspirin/magnesium stearate shows apparent melting onset at 66.38 °C, which is significantly lower than the aspirin and magnesium stearate individually. The presence of magnesium stearate accelerated the degradation of the aspirin. The change of the apparent melting temperature indicates the interaction between aspirin and magnesium stearate.
The Discovery X3 DSC is not only advantageous for clear comparison between the aspirin and magnesium stearate, but also provides a level of confidence in the interpretation of the results beyond that found on a single sample system. Since all 3 samples are run at the sample time it eliminates any change of the sample while waiting for the test. The Discovery X3 DSC also significantly increases the throughput for drug-excipient screening in pharmaceutical discovery and formulation development.
Long-Term compatibility Testing on X3 DSC
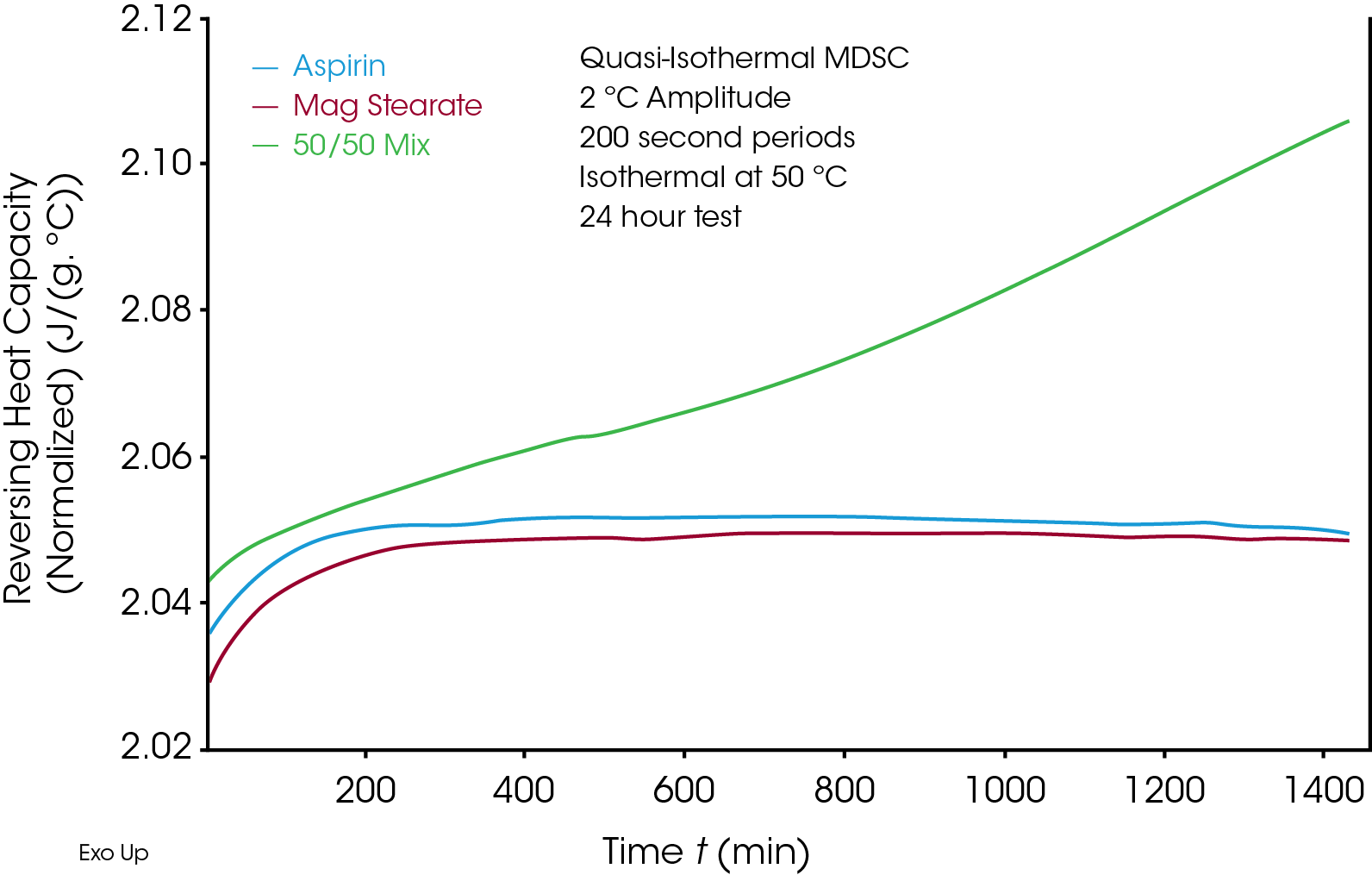
Long-term stability studies are important in the pharmaceutical industry to ensure formulated drugs are stable in long term storge conditions. These studies also provide useful information related to reaction kinetics, activation kinetics and reaction mechanisms. [3] A change in heat capacity is a good indication of any structural change in the sample. Amorphous structure typically has higher heat capacity than crystalline structure. Quasi-isothermal MDSC allows measurement of the heat capacity change over time at a set temperature. Figure 3 shows the measurement of heat capacity for the three aforementioned samples: Aspirin, Magnesium Stearate and 50/50 mixture of Aspirin/Magnesium Stearate. The heat capacity change is measured at 50 °C over 24 hours. The heat capacity of 50/50 mixture of Aspirin/Magnesium Stearate increased, indicating the structure of the sample changed over time, while Aspirin and Magnesium Stearate alone remain unchanged. The Discovery X3 DSC significantly reduced the instrument time to only 1 day, compared to a single sample DSC which would have required 3 days of instrument time. The Discovery X3 DSC significantly increases the throughput and provides fast turnaround for screening of drug-excipient incompatibility studies while maintaining high quality DSC data.
Conclusion
The Discovery X3 DSC featuring a multi-sample cell delivers high quality heat flow data for up to three samples simultaneously which can generate three times the amount of experimental data compared to a standard DSC. In these experiments with the Discovery X3 DSC all APIs, excipient and API-excipient mixture ran at the same time and under the same temperature profile. The Discovery X3 DSC significantly reduced run time while without sacrifice the high sensitivity in the data. Fast and high throughput screening for drug-excipient incompatibility is essential in pharmaceutical discovery and formulation development. The unique three sensor cell of the Discovery X3 DSC enables the fast turnaround needed to deliver the desired information.
References
- Aubuchon, S. R. and Thomas, L. C. A New Approach to Measurement of Drug-excipient Incompatibility. TA Applications Notes TA 348
- Thomas, L.C. and Schmidt, S. J. Apparent Melting”: A New Approach to Characterizing Crystalline Structure in Pharmaceutical Materials. TA Applications Notes TA 401
- Thomas, L. C. Apparent Melting: A New Approach to Detecting Drug-Excipient Incompatibility. TA Applications Notes TA 404
- Li, J. and Wu, Y. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants 2014, 2, 21-43; doi:10.3390/lubricants2010021
Acknowledgement
This note was contributed to by Len Thomas and Hang Kuen Lau, Ph.D., TA Instruments.
Click here to download the printable version of this application note.

