BioPharma Drug Development Workflow and Techniques
Julienne Regele | Calliste Scholl | Morgan Ulrich
November 27, 2023
Drug development is a long and complex process that starts with discovery and, if successful, ends with government approval for marketing. Each step in the drug development process, outlined below, has specific goals with the aim of down-selecting appropriate hits and candidates to an approved drug substance.
Here, we discuss the relevant objectives and techniques used in each stage of antibody drug development.
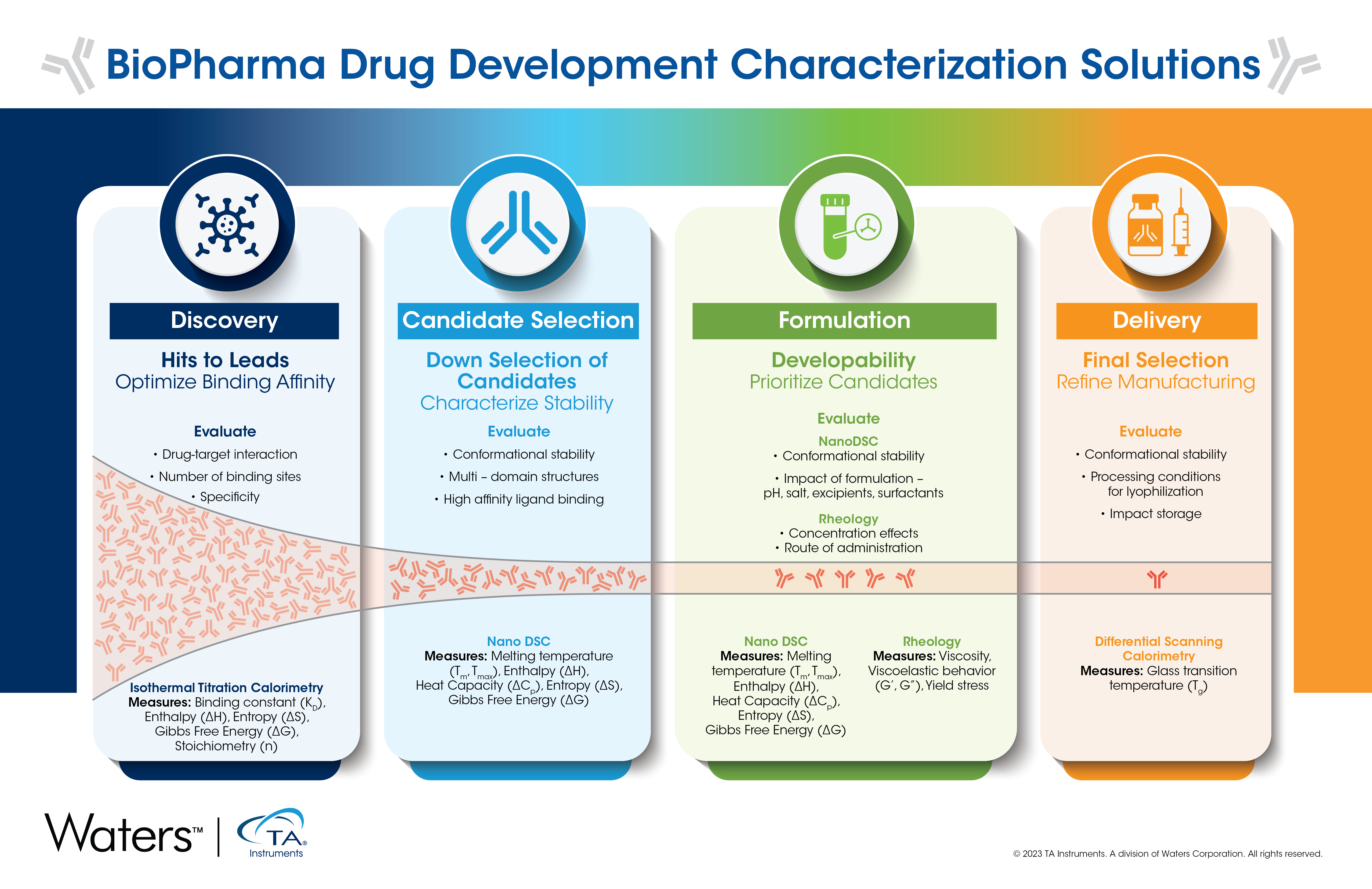
Discovery
The discovery phase is broken down into early and late-stage discovery. The primary goal of the discovery stages is to remove any sequence with undesired performance considering both chemical instabilities and target binding. Discovery will start with a large panel of antibodies that may range from hundreds to thousands and be reduced to a pool in the tens. To reduce the panel, in silico screening is commonly used as a primary tool because sample availability is very scarce at this stage. In silico screening uses computer simulations or virtual screening tools to make predictions about the behavior of different molecules.1 The sequence is analyzed to minimize the risk of any “hotspots” that might interfere with developability later in the process. Once the pool has been narrowed down, antigen binding is characterized to ensure the antibody binds to the identified target and the desired potency is also achieved again with the primary goal of reducing the number of antibodies to further characterize.
Pre-Formulation
At this stage, the number of antibodies has been reduced to the tens during the down-selection process. During pre-formulation, it is important to ensure tests are run under stable conditions, which means some level of buffer screening may be needed. Keep in mind, this buffer will likely differ from the final formulation buffer. It is important to fully characterize the remaining antibodies starting with validating binding studies carried out in initial stages with isothermal titration calorimetry (ITC).
ITC is a label- and immobilization-free method to characterize binding interactions. It is an information rich technique that not only tells if something binds, but also the driving forces behind the interaction. For any therapeutic, it is important to fully understand how it interacts with the target, including the specificity or how specific the therapeutic is to the target. Ensuring high specificity will result in a therapeutic with less undesired side effects caused by off-target binding. Other important attributes to characterize in this stage are the conformational stability, colloidal stability, potency, and plasma stability. This information will be used in combination to remove any unwanted antibodies.3
Candidate Selection
The candidate selection phase focuses on evaluating the antibodies that are most promising for therapeutic use. The techniques used in this section require concentrations that span over a larger range of up to 100 mg/mL and similar types of information will be collected, such as conformational stability and colloidal stability, however, this stage requires higher resolution data. Conformational stability will be assessed by characterizing both the Tonset and TM, using differential scanning calorimetry (DSC).
The automated Nano DSC allows researchers to characterize the short-thermal stability of their samples without the use of exogenous tags or dyes, thereby simplifying workflows and reducing errors in biopharmaceutical development.2 Within one experiment, researchers can determine the melting temperature, enthalpy, and heat capacity change, allowing them to calculate the free energy to make a fully informed decision on which formulation is most stable.2 This efficient determination facilitates the process of candidate selection. In addition, physicochemical characterization, in vivo PK/PD, and excipient assessment will also be completed.
Formulation
Once one or two antibodies are selected, the research moves to focusing on optimizing the final formulation for first in human trials. Conformational stability remains a critical attribute to measure and monitor through this formulation process. A series of formulations are created by varying pH, salt, sugars, and surfactants. Each combination is likely to change the conformational stability, so it is critical to measure stability. Unlike some of the other stages, it is important to conduct tests on the final antibody dosage, which could be up to 200+ mg/mL. The most valuable technique for stability testing is DSC, which provides accurate stability measurements to compare different formulations to see which has the highest potential for developability.2
Delivery
Another key area to consider during the formulation and/or commercial formulation process is the mode of delivery. The delivery mode will influence the final dosage and the final form of the product, liquid or lyophilized. The two most common delivery methods for antibody drug formulations are liquid stable subcutaneous injections or lyophilized and reconstituted solutions for intravenous infusion.4 If the formulation is lyophilized to improve shelf-life, then the conditions under which to lyophilize can be better understood by determining the glass transition temperature or collapse temperature.5 During the primary drying step, it is critical to design a temperature ramp that stays below this temperature to avoid structure collapse or shrinkage. Differential scanning calorimetry (DSC) is the gold standard and preferred tool to understand this information.
Cutting-Edge Microcalorimeters for Expedited Drug Development
While the drug development process is long and arduous, TA Instruments provides efficient, user-friendly solutions to facilitate research, development, and manufacturing. TA collaborates closely with our customers to determine the best solution that is fit for purpose in each of the drug development milestones. Our range of microcalorimetry equipment is specifically designed to grow with your research needs with the capability to add on auto sampling at any point. And, TA Instrument’s long-standing history of sensitive equipment ensures credible, reliable data.
References
- In silico pharmacology for drug discovery: Methods for virtual ligand screening and profiling—PMC. (n.d.). Retrieved October 20, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1978274/
- Nano DSC – TA Instruments. (2023, March 19). https://www.tainstruments.com/nanodsc/
- Affinity ITC – TA Instruments. (n.d.). Retrieved October 20, 2023, from https://www.tainstruments.com/affinity-itc-auto/
- Belissa, E., Vallet, T., Laribe-Caget, S., Chevallier, A., Chedhomme, F.-X., Abdallah, F., Bachalat, N., Belbachir, S.-A., Boulaich, I., Bloch, V., Delahaye, A., Depoisson, M., Wojcicki, A. D., Gibaud, S., Grancher, A.-S., Guinot, C., Lachuer, C., Lechowski, L., Leglise, P., … Boudy, V. (2019). Acceptability of oral liquid pharmaceutical products in older adults: Palatability and swallowability issues. BMC Geriatrics, 19(1), 344. https://doi.org/10.1186/s12877-019-1337-2
- Affairs, O. of R. (2022). Lyophilization of Parenteral (7/93). FDA. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/lyophilization-parenteral-793
Other Resources
- Application Note – Characterizing Virus Structure and Binding
- Application Note – Characterizing Protein – Protein Interactions by ITC
- Application Note – Characterization of Biopharmaceutical Stability
- Application Note – Advances in Native Binding Assays
- Application Note – Determining Thermal Stability of Antibodies with a Nano DSC
- Application Note – A Novel Thermodynamic Assay for Predicting and Monitoring Biomolecular Structure Stability
- Webinar – Biophysical Characterization of Antibodies in a Suspension: Solutions for Slurries
- Webinar – Biophysical Characterization of Antibody Drug Conjugates Using DSC
- Instrument – Nano DSC
- Instrument – Isothermal Titration Calorimeters (ITC)
- Instrument – Isothermal Microcalorimeters
- Contact – Contact TA Instruments Today