Keywords: Gene therapy, viral vector, AAV, microcalorimetry, differential scanning calorimetry (DSC)
MC161
Abstract
Gene therapy is a rapidly developing area of research with the potential to save lives by curing previously uncurable diseases. A growing focus has been put on developing viral-based vectors to aid in the delivery of these therapies. Adeno-associated viruses (AAVs) are a leading gene therapy platform but pose challenges regarding encapsidation efficiency and quality control. This work highlights the use of the Nano DSC to overcome some of the challenges associated with AAVs.
Introduction
Gene therapy seeks to cure disease by introducing genetic material to a patient’s cell that can modify gene expression. A key challenge associated with gene therapy development includes identifying an appropriate drug-delivery vector that can direct the therapy to the target cell. To this end, viral-vector-based therapies represent a growing number of FDA approved gene therapies on the market. As of early 2022, eight viral-vector-based gene therapy products gained FDA approval1 and, with at least 25 more viral vectors in late-stage development, this number is expected to grow.2
Adeno-associated viruses (AAVs) represent a class of viral-vector-based gene therapy product that has received considerable attention. These virus-like vectors are a protein-based delivery system that can efficiently traverse cell membranes. For this reason, AAVs are a leading platform of gene delivery vehicles.3
Despite their rapid growth, the use of AAVs for gene therapy is not without challenges.4 The industry is increasingly moving towards mass production of viral-vector-based gene therapies, but this poses risks to AAVs. Harvested from cell cultures, production scale-up of AAVs may lead to contamination with residual byproducts, such as proteins, nucleic acids, and bacteria. To combat this, manufacturers need to employ methods to confirm the integrity of AAV gene therapy. Additionally, AAVs need to be properly loaded with the intended gene therapy, and quality control methods need to be developed to determine whether the vector is empty or full. The nano DSC from TA Instruments offers solutions to these challenges.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) is a powerful biophysical characterization technique used to describe a macromolecule by its enthalpy (ΔH) and TM (ΔG relationship). Its ability to measure small variations in heat capacity of a sample that may result from changes to the sample’s structure make DSC a popular technique for investigating other drug delivery vehicles.5 The nano DSC can detect changes in intermolecular interactions that may result from changes in AAV structure due to temperature, in addition to transitions caused due to contaminants, storage and buffering conditions, formulation changes, and batch to batch variability. Due to the broad range of experiments it can accommodate, the nano DSC can provide workflow solutions at many stages of gene therapy development, from modulating drug release characteristics of the AAV to monitoring batch to batch variability during production.
Experimental
Each sample was loaded manually into the Nano DSC and scanned once from 50 °C to 110 °C at a scan rate of 1 °C/min. Data analysis was performed using the accompanying NANOANALYZE software version 3.12.0.
Results and Discussions
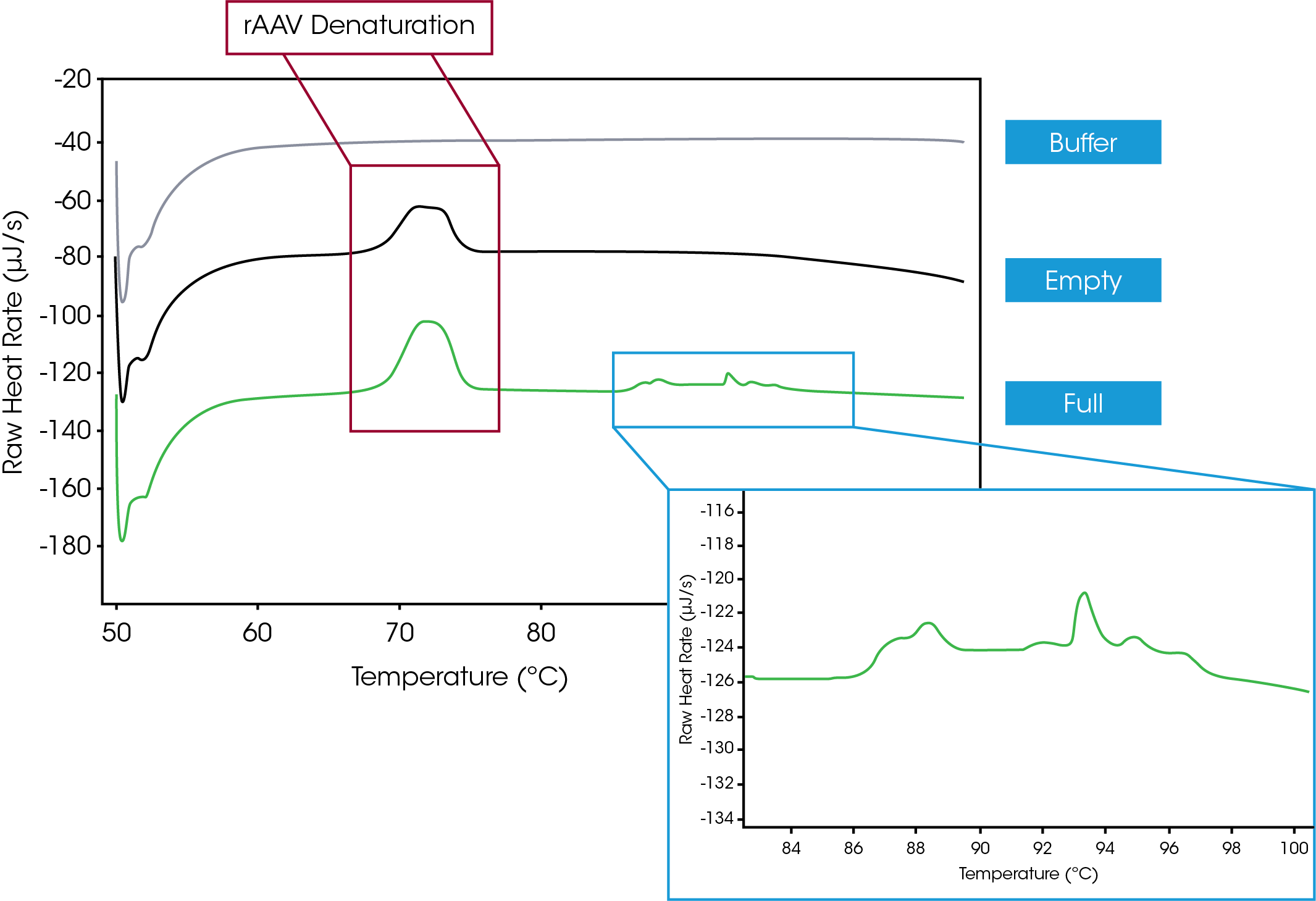
Initial efforts to gain thermodynamic information about AAVs involved testing the capsids with and without a nucleic acid payload. Figure 1 shows a large thermal event beginning at 70 °C that is indicative of AAV denaturation. An additional series of thermal transitions, shown in the Figure 1 inset, start at 86 °C, and are representative of DNA that was released from the AAVs upon thermal degradation. Finally, analysis with NanoAnalyze software demonstrates that the AAV gains 0.3 °C of thermal stability when full compared empty, which allows the interaction of the vector and its payload to be characterized. Together, these findings highlight the ability of the nano DSC to evaluate the differences between empty and full viral vectors.
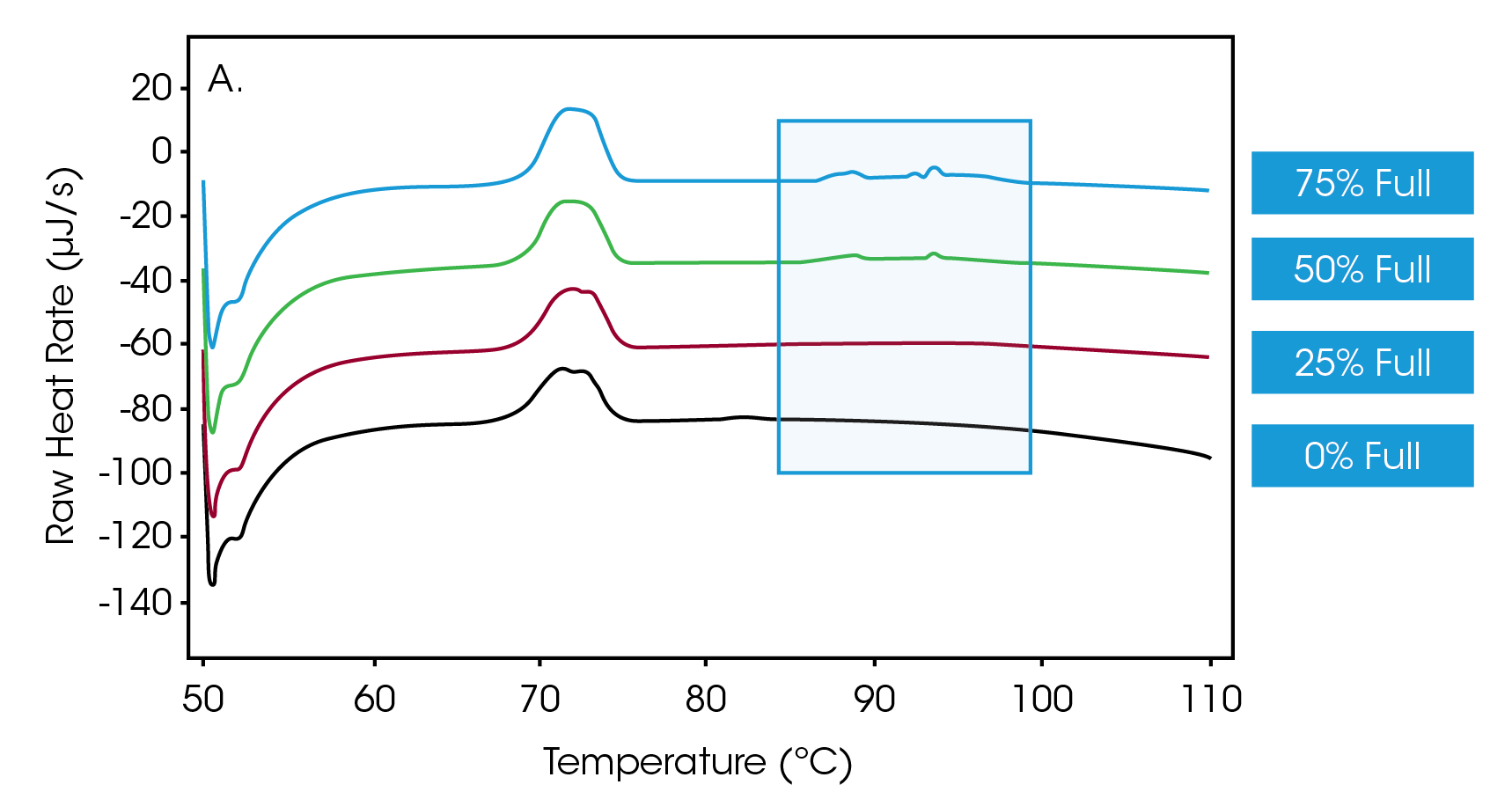
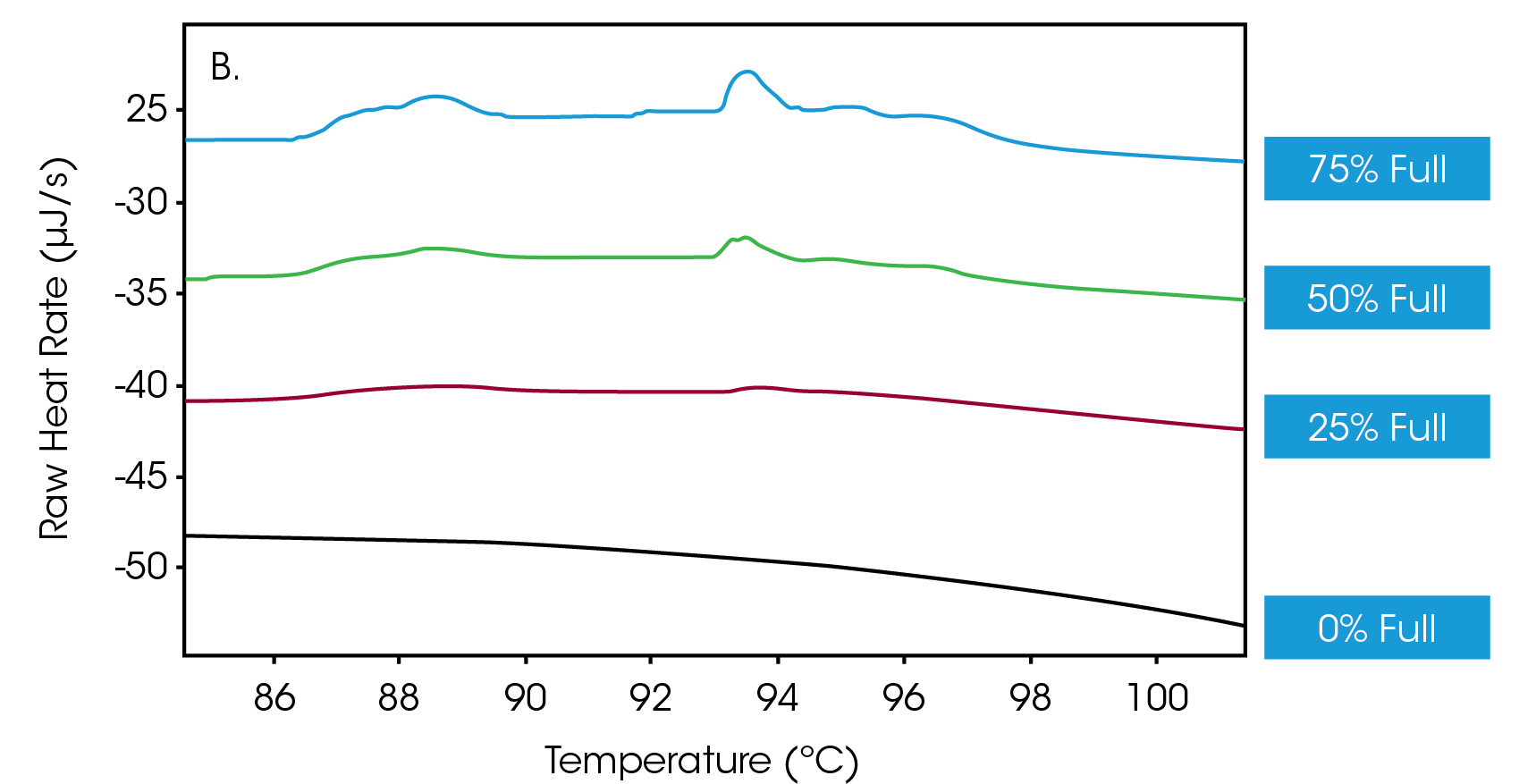
Next, efforts were directed towards determining whether the Nano DSC could discern different ratios of empty-versus-full capsids. Accordingly, solutions of full capsids were doped with empty capsids to achieve a mixture of AAVs in which 75%, 50%, 25% and 0% of the vectors were full. Figure 2A shows a primary thermal event beginning at 70 °C that represents the melting of the AAV capsid, while Figure 2B shows an enlarged view of the transitions, indicative of DNA denaturation. These thermograms showcase that the nano DSC can detect drug product when the encapsulation efficiency is as low as 25%.
Conclusions
The Nano DSC is a powerful analytical tool that can be used to ensure integrity and quality of AAVs for gene therapy. It can evaluate stability of gene therapy vectors, encapsulation efficiency, and the interactions between the vector and its payload. Additionally, this method can be adapted to optimize storage conditions, batch to batch variability, and release of the drug product.
References
- Approved cellular and gene therapy products, US Food and Drug Administration, updated June 23, 2022, fda.gov.
- EvaluatePharma®, February 2022, Evaluate Ltd.
- Wang, D., Tai, P.W.L., Geo, G., (2019). Adeno-associated virus vector as a platform for gene therapy delivery, Nat. Rev. Drug Discov, 18, 358-378.
- Hebben, M. Downstream bioprocessing of AAV vectors: industrial challenges & regulatory requirements. 2018, Cell Gene Ther. Ins. 4(2), 131-46.
- Quinn, C., Shion, H. Antibody Drug Conjugate (ADC) load characterization and analysis using the BioAccord and Nano DSC systems. (2020) Waters Corporation Application Brief.
Acknowledgement
This paper was written by Samuel Redstone, PhD, Applications Scientist at TA Instruments.
Click here to download the printable version of this application note.

