Keywords: TGA, DSC, Degradation, Melting, Polymer
TA430
Introduction
Polymer degradation occurs when polymeric materials undergo elevated temperatures that can cause the loss of physical properties, such as optical and mechanical properties. Common polymer processing techniques, for instance extrusion, hot press, injection molding, and blow molding often involve heating the polymer to above the melting point for ease of the processing. The temperature required for processing should be above the melting temperature and below the degradation temperature of the polymer. However, polymers under elevated temperatures could be slightly degraded, and it may be difficult to characterize the minor degraded polymers.
In this Applications note, we demonstrate using TGA to precisely control the degree of degradation to be < 5wt% and characterize the impact of degrees of degradation on polymer thermal properties. Commonly used semi-crystalline polymer Nylon 6,6 was chosen and tested in this study with its well-known glass transition and melting temperature.[1]
Experimental
In this Applications note, we demonstrate using TGA to precisely control the degree of degradation to be < 5wt% and characterize the impact of degrees of degradation on polymer thermal properties.
Determine the Moisture Content and Degradation of Nylon 6,6
Purge gas: Nitrogen
Procedure:
1: Ramp 10 °C/min to 1000 °C
TGA was used to remove moisture and to generate the degraded Nylon 6,6 samples. Utilizing the Abort segment, the Nylon 6,6 sample can be degraded precisely from 0% to 4% in TGA.
Remove moisture from Nylon 6,6
Purge gas: Nitrogen
Procedure:
1: Ramp 10 °C/min to 300 °C
Control the Degrees of Degradation of Nylon 6,6 with TGA
Purge gas: Nitrogen
Procedure:
1: Abort if wt% is < desired wt% (Table 1)
2: Ramp 10 °C/min to 1000 °C
The impact of the degrees of degradation on the thermal properties of Nylon 6,6 were studied using a heat-cool-heat experiment on a Discovery DSC 2500.
DSC Heat -cool-heat Experiment
Purge gas: Nitrogen
Procedure:
1: Equilibrium at 0 °C
2: ramp 10 °C/min to 300 °C
3: ramp 10 °C/min to 0 °C
4: ramp 10 °C/min to 300 °C
Results & Discussion
Thermal Degradation of Nylon 6,6
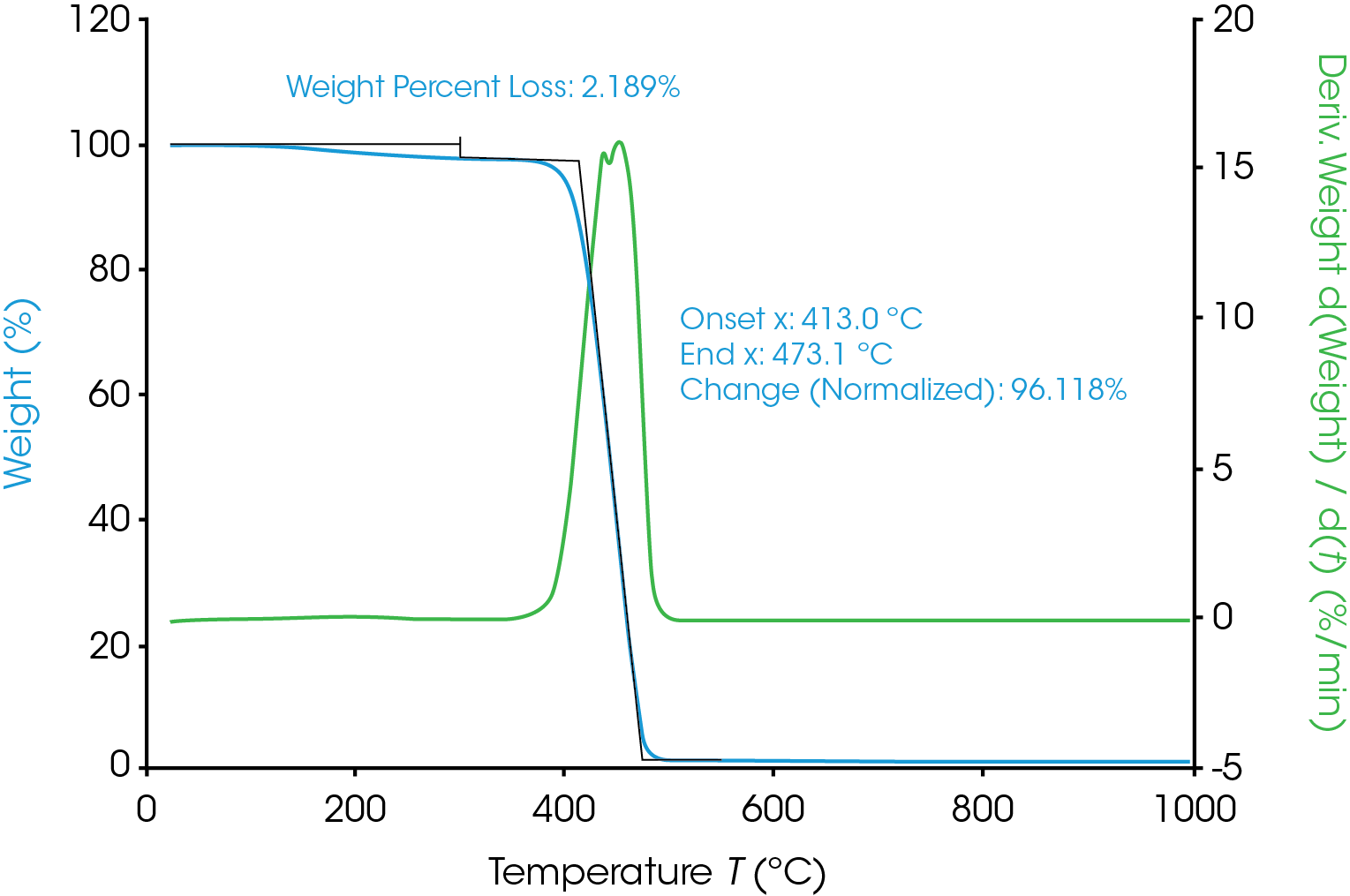
TGA was used to determine the moisture content and degradation profile of the Nylon 6,6. As shown in Figure 1, the moisture content of the Nylon 6,6 sample was approximately 2.2%. The degradation onset temperature was around 413 °C and the weight loss at the onset temperature was 96.1%.
Control the Degree of Degradation with TGA
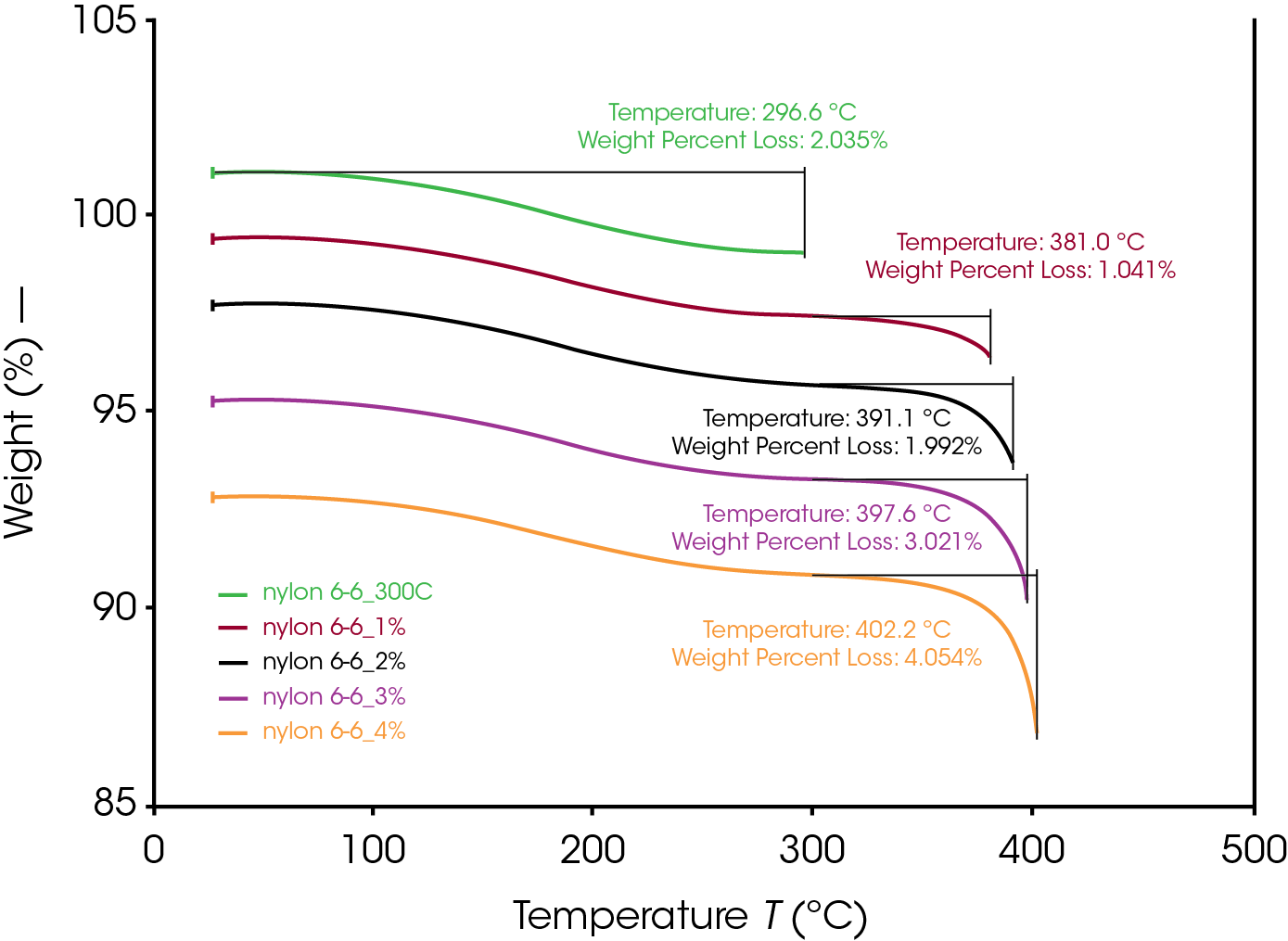
Even below the degradation onset temperature, polymers could still be slightly degraded (<5 wt% degradation). To study the effects of small degrees of degradation of polymer on their thermal properties, TGA was to precisely control the degrees of degradation. Nylon 6,6 was heated to above 300 °C to remove the ~2% moisture. With the Abort segment, TGA can be used to generate desired thermal degraded sample with 1% to 4% of degradation. The procedure of the abort segment was set to be between 97-94% as stated in Table 1 to account for the ~2% moisture content from the Nylon 6,6. The degree of degradation was determined from the wt% loss from 300 °C to maximum temperature as shown in Figure 2. As it can be seen, TGA enabled a precise control of degrees of degradation.
Table 1. TGA Controlled Degrees of Degradation
| Abort Procedure (<wt%) | Degree of Degradation (wt%) | Maximum Temp (ºC) | |
|---|---|---|---|
| Nylon 6,6 | — | 0 | — |
| 300 °C | — | 0 | 296.6 |
| 1% | 97% | 1.041 | 381.0 |
| 2% | 96% | 1.992 | 391.1 |
| 3% | 95% | 3.021 | 397.6 |
| 4% | 94% | 4.054 | 402.2 |
Degradation Impact on Thermal Properties
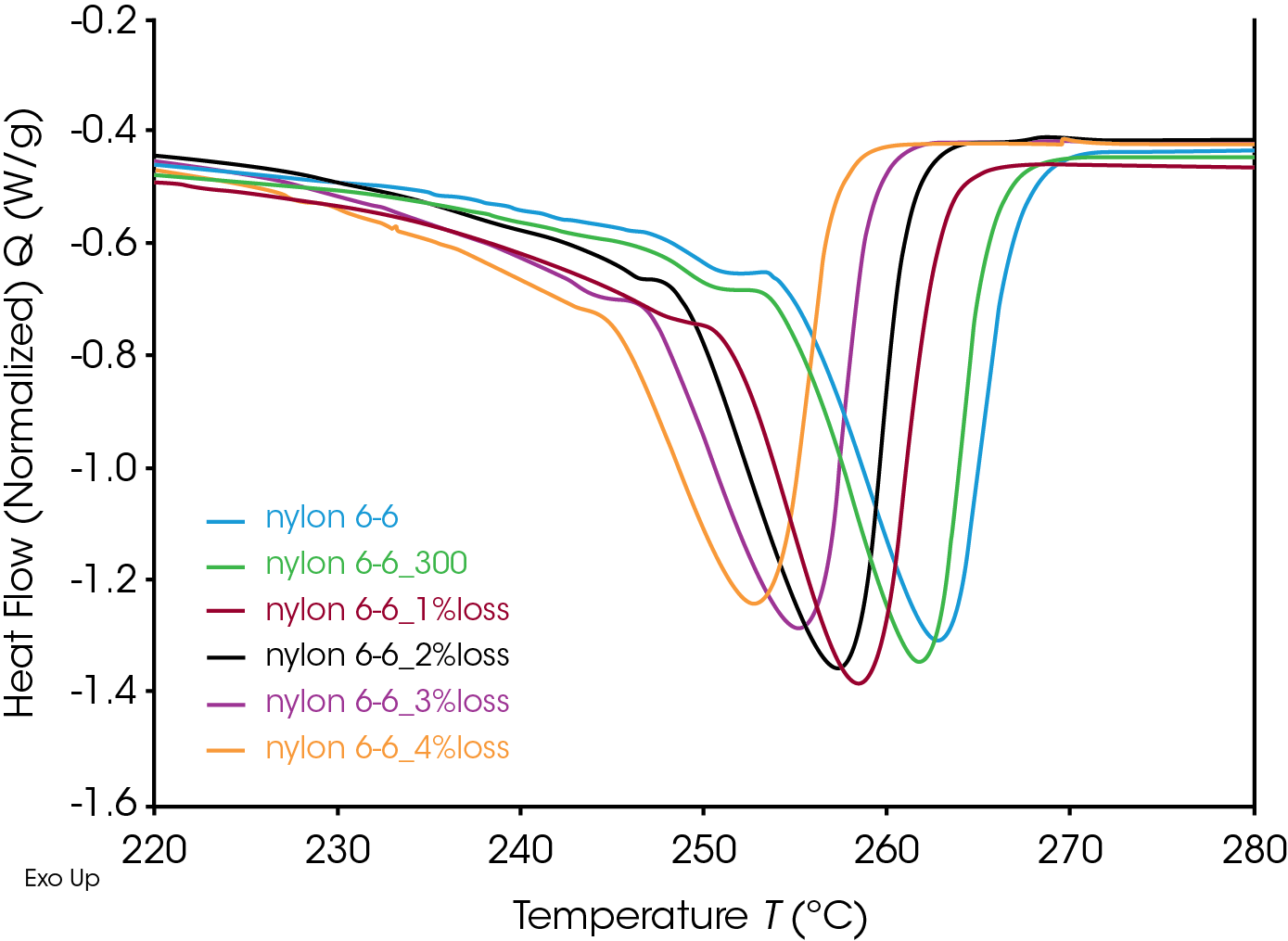
During thermal degradation, random-chain scission, end-chain scission and side-chain cleavage can occur.[2] Chain scission will reduce the molecular weight and change the molecular weight distribution of the polymer and hence will significantly affect the viscosity and flow properties; and further affecting on the quality of the polymer processes. The thermal properties of a polymer (such as glass transition and melting) are highly sensitive to the molecular weight and molecular weight distribution.[3] Hence DSC can be a simple way to characterize the degree of degradation of a polymer. The degraded Nylon 6,6 samples were tested on a DSC 2500 to study the thermal properties. A heat-cool-heat experiment was performed from 0 °C to 300 °C with 10 °C/min heating and cooling rates. The first heating removes any thermal history of the samples. To give all the samples the same thermal history, 10 °C/min cooling rate was used for cooling and the second heating curves were used for all the data analysis and comparisons.
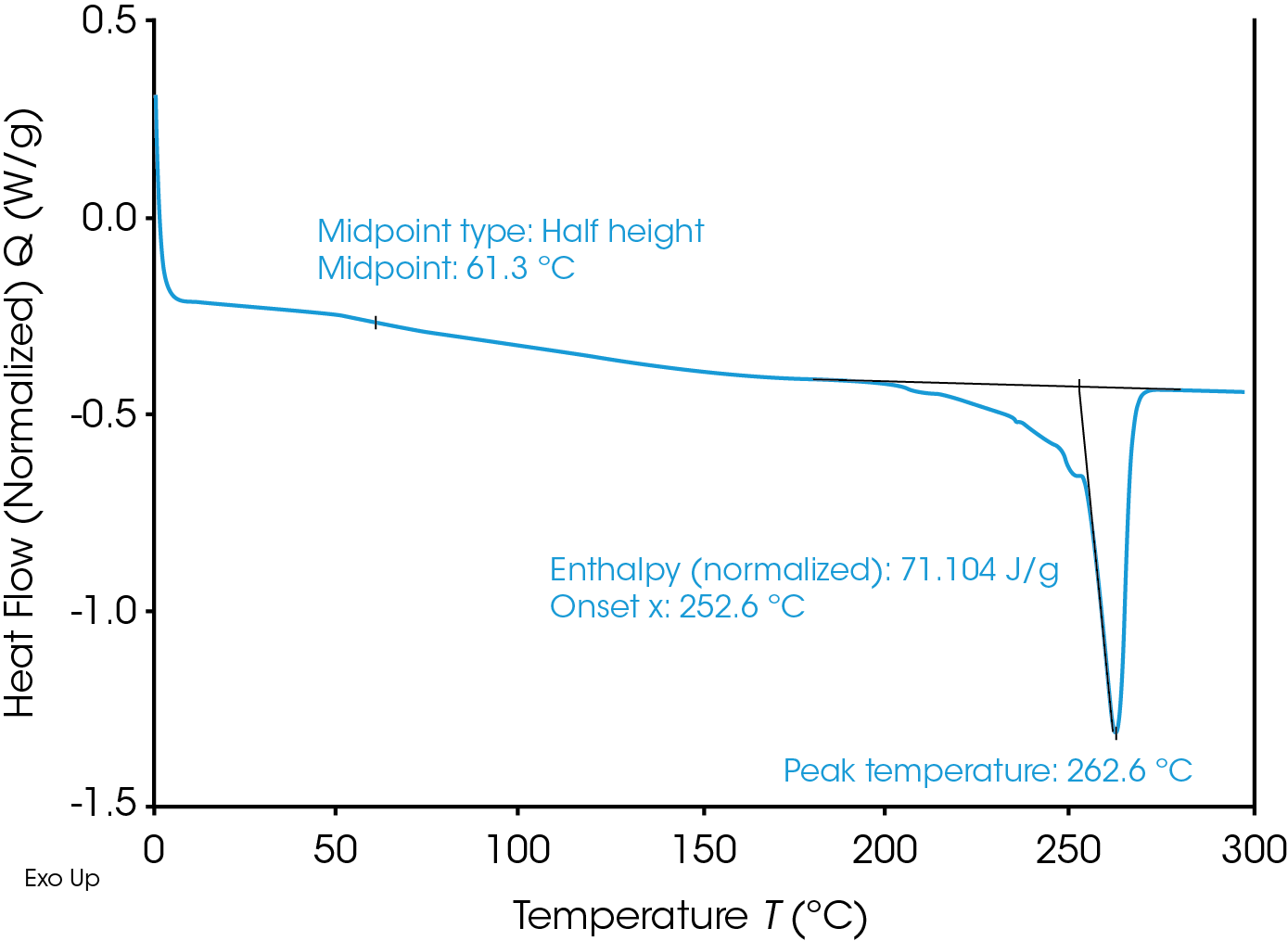
Glass transition and melting were observed in the DSC thermograph. Data analysis was performed using “Glass Transition” and “Peak Integration (enthalpy)” analysis functions in TA Instruments TRIOS software. Figure 3 shows the analysis results of the untreated Nylon 6,6 sample. The Nylon 6,6 shows a glass transition at 61.30 °C and a melting peak at 262.6 °C.
Nylon 6,6 samples with removed moisture and controlled degrees of degradation using TGA were also tested using DSC. All the data was used the same analysis for comparison and the results are shown in Table 2.
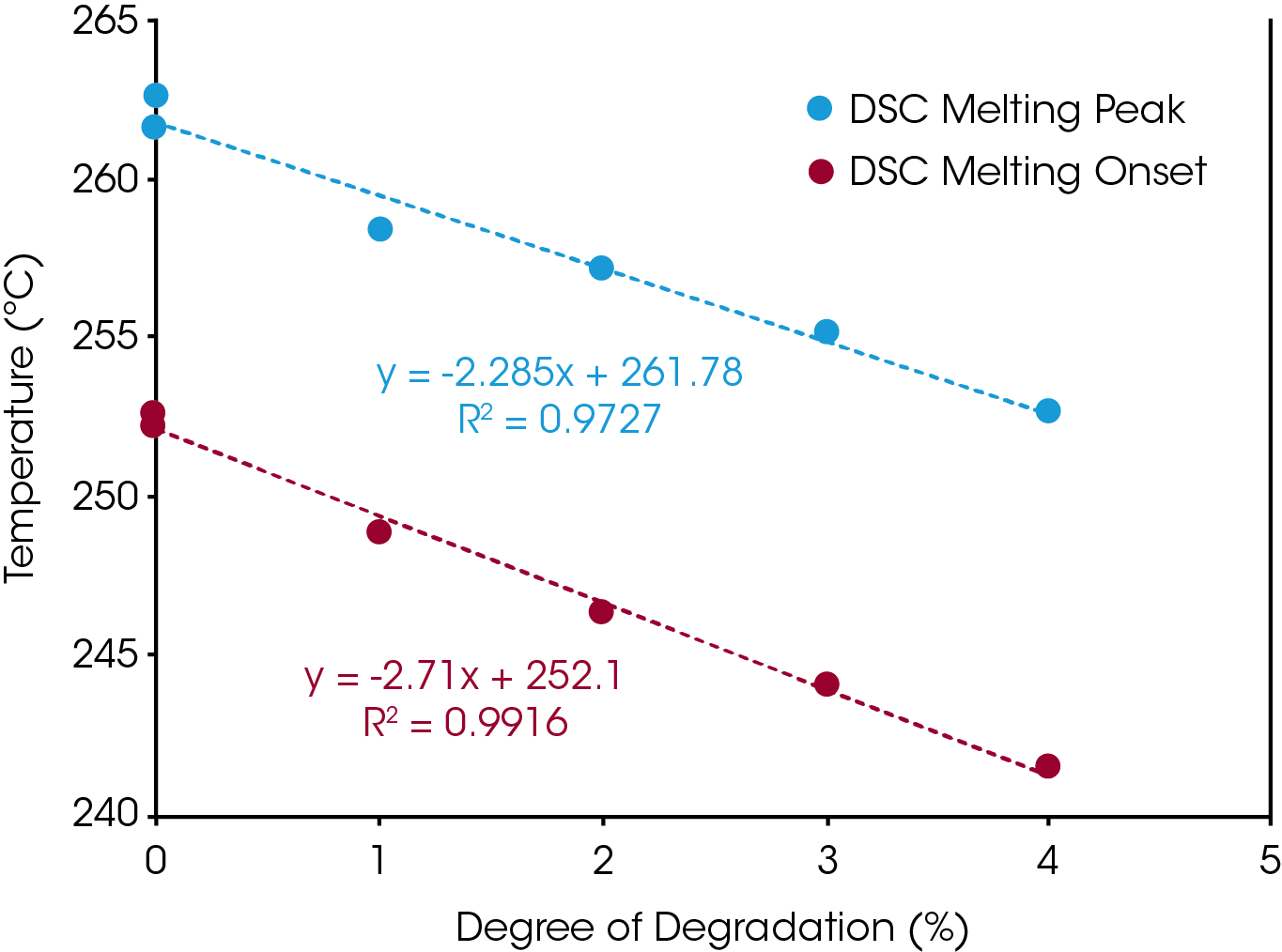
Table 2. Degree of Degradation Impacts on the Glass Transition and Melting of Nylon 6,6
| Glass Transition (ºC) | Melting Onset | Maximum Temp (ºC) | |
|---|---|---|---|
| Nylon 6,6 | 61.3 | 252.6 | 262.6 |
| 300 °C | 60.53 | 252.2 | 261.7 |
| 1% | 58.41 | 248.8 | 258.4 |
| 2% | 57.46 | 246.3 | 257.2 |
| 3% | 56.81 | 244.1 | 255.2 |
| 4% | 52.21 | 241.5 | 252.7 |
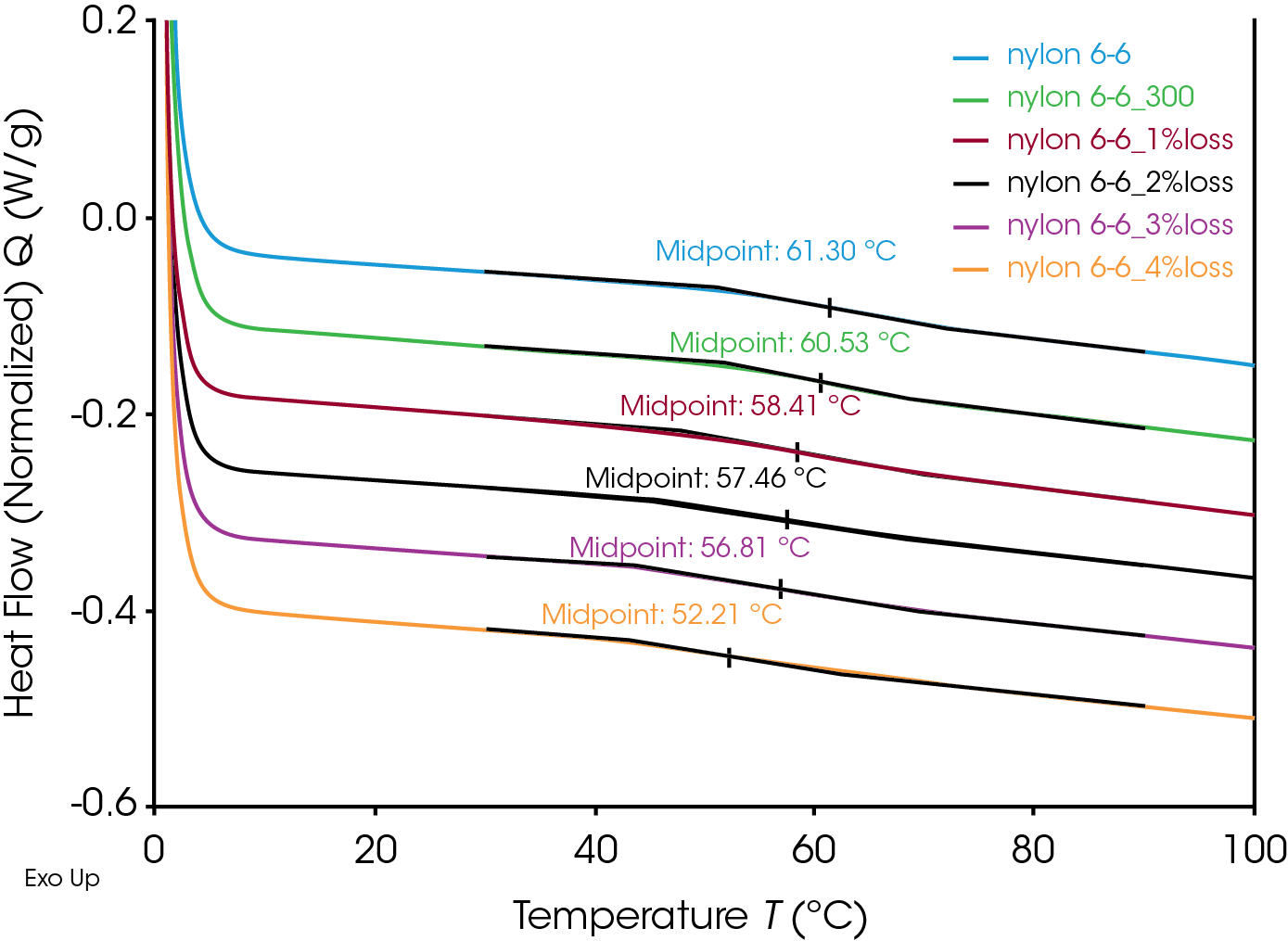
The glass transitions of the degraded Nylon 6,6 samples shifted to lower temperatures with increasing degrees of degradation (Figure 4). The decrease in glass transition may be due to the chain scission and the reduced molecular weight during the thermal degradation. Straus et. al. showed random chain scission was predominate in Nylon decomposition.[4] Random chain scission resulted in lower molecular weight species and increase molecular weight distribution. The molecular weight reduction enhanced the molecular mobility of the polymer chain and therefore reduced the glass transition temperature. The same trend was also observed in the melting temperature. The melting onset and peak temperatures decrease with increasing degrees of thermal degradation (Figure 5). The Nylon 6,6 melting data can be fitted into a linear fitting (Figure 6) and used as a calibration curve to further quantify the degree of degradation of an unknown samples. The correlation between the thermal properties and degrees of degradation can be studied for different materials with TGA which provided a precisive controlled degradation.
Conclusion
The Discovery DSC™ 2500 & TGA 5500 are complimentary tools that allow the determination of moisture content, thermal stability, and the effect of degradation on the thermal properties. The TGA is able to precisely control the degree of degradation, while the DSC can study this effect on the thermal properties. The glass transition and melting peak temperature of Nylon 6,6 are lowered with increasing degrees of degradation. The same study can be applied to different polymer system with Discovery TGA precisely control the degree of degradation to understand degradation impact on materials thermal properties.
References
1. Common Polymers Reference Card, TA Instruments (2015)
2. Beyler, C. L. and Hirschler, M. M. Thermal Decomposition of Polymers
3. Blaine, R. L. and Waguespack, L. E. Determination of Polymer Crystal Molecular Weight Distribution by DSC. TA Instruments Applications Notes TA 276
4. Straus, S. and Wall, L. A. J. Res. Natl. Bur. Stand. (U. S.) 60, 39 (1958)
Acknowledgement
This note was written by Hang Kuen Lau, Ph.D., TA Instruments.
Click here to download the printable version of this application note.

