Keywords: TGA, glovebox, inert environment, lithium-ion batteries, water sensitive materials, oxygen sensitive materials
TA471
Abstract
Many materials, including those found in lithium-ion batteries, are reactive with atmospheric components. Testing and handling of such materials must be done in an inert environment, such as a glovebox. It can be challenging but necessary to install analytical instruments in a glovebox to provide an inert testing environment as in many cases, sample integrity is compromised by a brief exposure to nitrogen, oxygen, or water. This note illustrates the advantages of working with a thermogravimetric instrument in an inert environment by way of a simple example with a water sensitive material.
Introduction
Certain materials must be handled and tested within an inert environment. Frequently, thermal analysis work is performed with inert purge gases supplied to furnaces or cells, thus affording an inert testing environment to a high degree. Thermogravimetric analysis (TGA) instruments, which measure the mass change of a sample over time and temperature, can operate with inert purges. Some TGA pan designs also allow for sealing and then opening just prior to loading, thereby protecting sensitive materials, and enabling the queuing of multiple samples on autosamplers. Issues may still arise with highly sensitive samples in the short period of time from pan opening to loading within the furnace or cell. Working with extremely sensitive samples usually requires the analytical instrument to be installed within a glovebox so sample preparation and autosampler queue time all occur within an inert environment.
Many lithium-ion battery (LiB) materials fall into the category of highly sensitive and reactive materials. Samples can be sensitive to nitrogen, oxygen, and water, and thus are typically tested within gloveboxes filled with argon. The common electrolyte lithium hexafluorophosphate (LiPF6) must be studied in a water free environment. Decomposition of anhydrous LiPF6 should occur in one step (LiPF6 → LiF + PF5), while the presence of water causes additional reactions, and these reactions may produce hydrogen fluoride (PF5 + H2O → POF3 + 2HF). As measured by the TGA, the decomposition temperature of the hydrolyzed samples is reduced compared to anhydrous samples, potentially yielding misleading results [1]. Another battery material, pure lithium, must be protected from interaction with nitrogen, oxygen, and water as it easily reacts with all three of these at room temperature [2]. Some samples are so sensitive that brief exposures to any of these gases can be detrimental to the sample integrity and subsequent data collected. This sensitivity negates the use of sealed pans that are opened just prior to insertion into the analytical instrument.
TA Instruments recognizes these challenges and has introduced appropriate accessories to make installation of instruments, including TGAs, within a glovebox straightforward. This note illustrates the protection provided by working in an inert environment.
Application Benefits
- Atmospheric-sensitive samples can be challenging to work with. Many types of benchtop analytical instruments are being installed into gloveboxes to address this problem. However, this can be a challenge in itself.
- TA Instruments has developed hardware that makes installing a TGA within a glovebox a simple task, thereby making this solution more attractive.
Experimental
Desiccant material, which readily absorbs water, was used to illustrate the advantages of working in a glovebox environment. Indicating DRIERITE™ from W. A. Hammond Drierite Co. LTD, composed of ≥98% CaSO4 and <2% CoCl2 [3] will change from blue to pink upon the absorption of moisture.
Identical experiments were performed on two TA Instruments™ Discovery™ 5500 TGAs, using 100 µL open platinum pans and nitrogen purges. One TGA was in ambient lab conditions, the other installed in a glovebox purged with nitrogen. For both experiments, a single granule of DRIERITE was tested using the follows steps:
- Sample was heated to 150 °C at 10 °C/min and held isothermal for one hour to remove any absorbed moisture. Samples were then unloaded from the TGA onto the autosampler tray.
- The sample was allowed to sit in the autosampler tray for various amounts of time: 1, 10, 30, 60, 120, 180, and 300 minutes. It should be noted that the times listed only indicate the time spent sitting on the autosampler and do not include instrument loading and unloading times. As expected, the sample studied at ambient conditions progressively changed from blue to pink the longer it was exposed to the atmosphere. The sample studied inside the glovebox remained blue.
- After being held on the autosampler for the prescribed amount of time, samples were again loaded and ramped to 150 °C at a rate of 10 °C/min and held for one hour at temperature. This step measures the amount, if any, of moisture absorbed while sitting on the autosampler.
The glovebox with instrument inside is shown in Figure 1. For a TGA, items such as power cords, purge gas lines, water cooling lines and communications cables must be passed through from the outside to the inside of the glovebox. This is easily accomplished with the use of flanges and appropriate passthroughs, available in a dedicated kit from TA Instruments. This hardware kit makes installation of a TGA in a glovebox straightforward and reliable.

Results and Discussion
Figure 2 shows the results from the TGA in ambient lab conditions, outside a glovebox. The moisture absorption experiments are overlayed together and identified in the legend. The residual weight for each run is measured. As an indication of the sensitivity of the desiccant granule, the sample has absorbed moisture after resting on the autosampler for only a minute. As the exposure time increases, the moisture content also increases.
Plotting the data as micrograms versus time, as shown in Figure 3, confirms that the sample is indeed gaining weight and, upon heating, always returns to the initial dry weight to better than 0.05%.
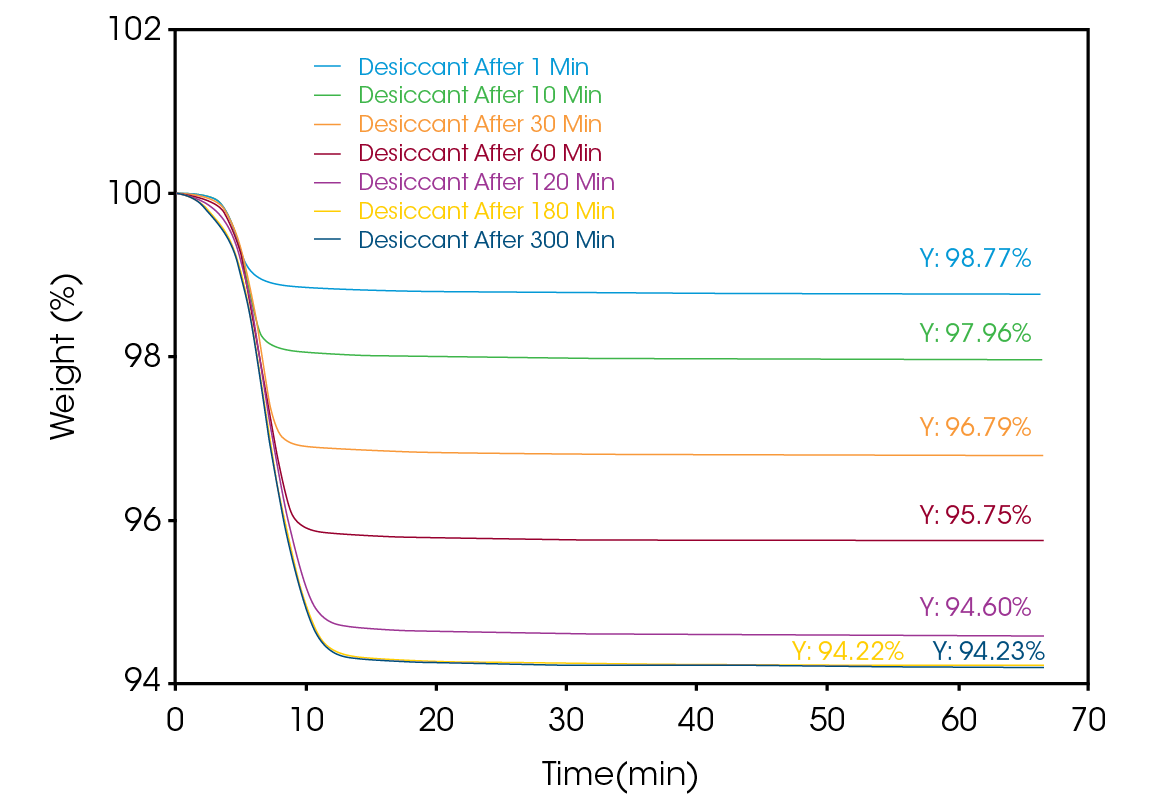
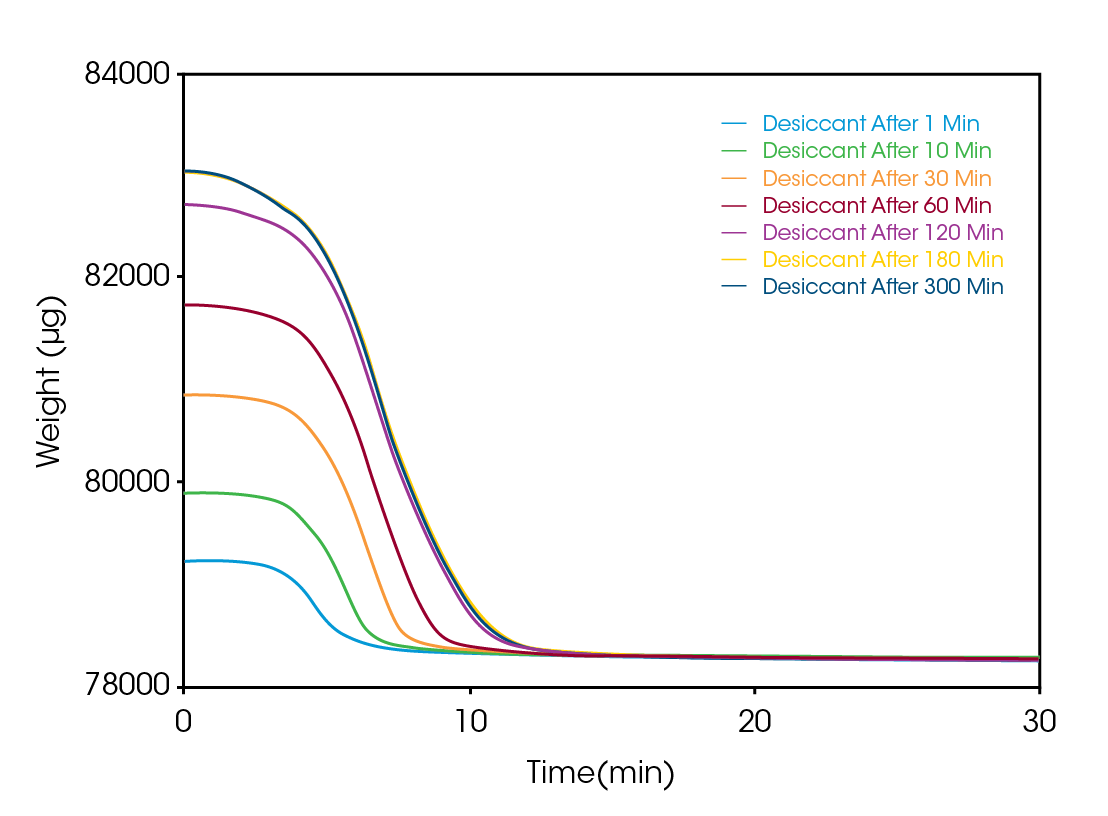
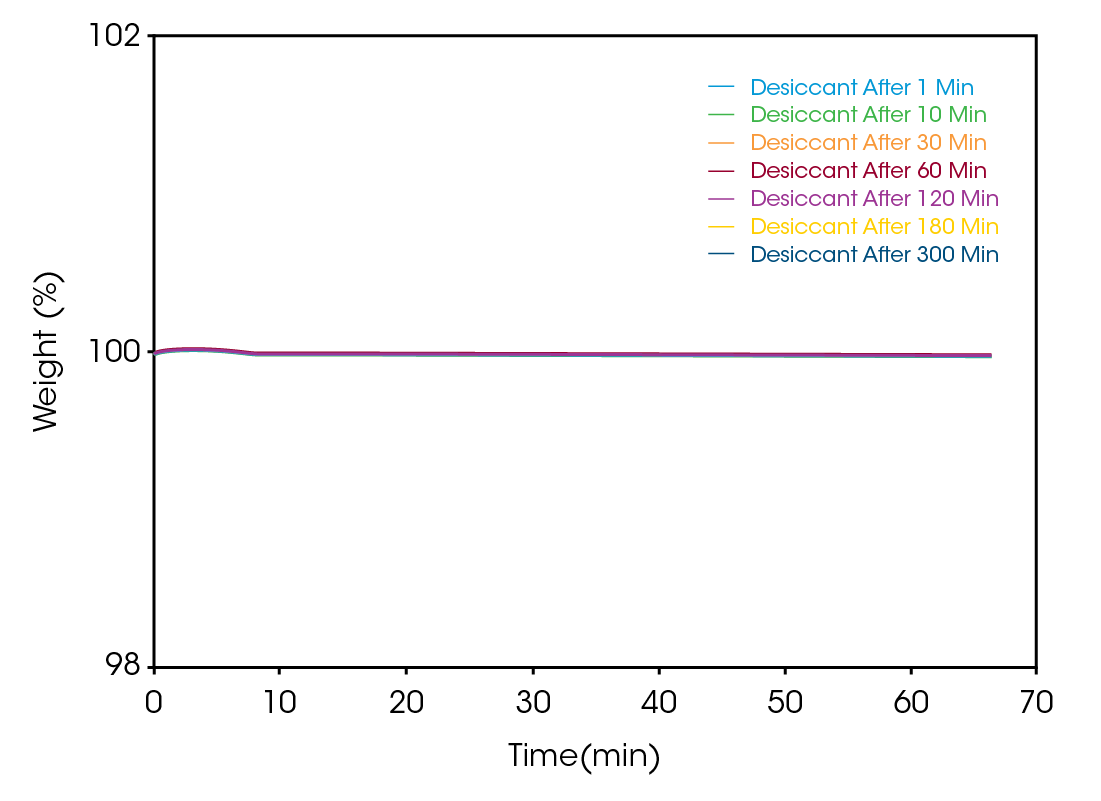
Figure 4 shows the results of samples handled and tested within the glovebox, yielding different results to the samples run in ambient conditions. The data from increasing time periods of the sample on the autosampler is plotted. However, here, because the environment is dry, the sample weight is essentially stable up to a time of 300 minutes on the autosampler. No measurable trend in the data is detected that would indicate even small amounts of moisture being absorbed.
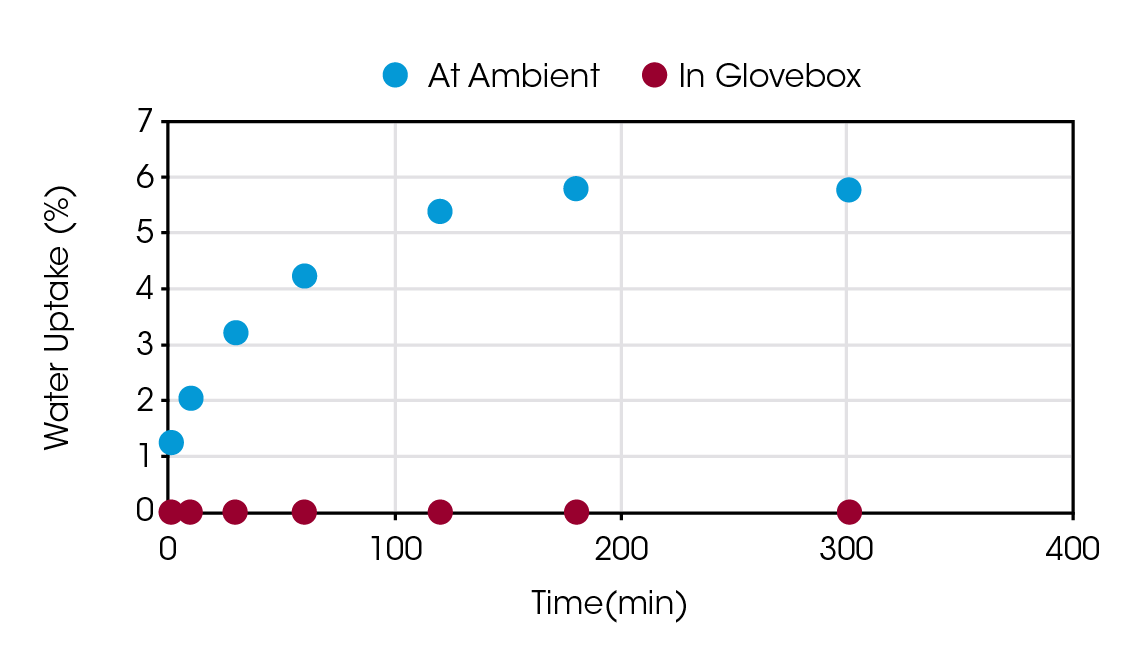
Figure 5 shows an overlay scatter plot of moisture uptake versus time. It is obvious that the sample at ambient conditions has saturated its moisture uptake at approximately the three-hour mark. Conversely, data within the glove box indicates a sample that shows essentially no water uptake.
The data shown indicates that in a glovebox, a sample can sit on the autosampler for up to 300 minutes with no impact on its integrity. An additional run within the glovebox was performed after 24 hours, or 1440 minutes, that showed a weight increase of 0.057%. For runs up to 1000 °C at 10 °C/min on the Discovery 5500, 1440 minutes would encompass 14 queued runs on the autosampler. At 20 °C/min, more than 25 runs are possible, which is the autosampler tray maximum.
Conclusions
Working in a glovebox environment can act to protect highly sensitive samples, such as those commonly found in LiB research. The TGA Glovebox Adaptor Kit from TA Instruments simplifies the process of installing a TGA in a glovebox, which allows for easier and more efficient analyses of these atmospherically sensitive materials.
DRIERITE desiccant was used to demonstrate the importance of environmental control for handling and testing TGA samples that are sensitive to environmental conditions. The results clearly revealed moisture uptake differences in samples run within a nitrogen-controlled glovebox versus in ambient conditions. The data presented in this note indicate how, with a properly installed instrument in a glovebox, no special precautions are needed to protect sensitive samples waiting to be tested on the autosampler.
References
- L. Kock, M. Lekgoathi, P. Crouse and B. Vilakazi, “Solid State Vibrational Spectroscopy of Anhydrous Lithium Hexafluorophosphate (LiPF6),” Journal of Molecular Structure, pp. 145-149, 2012.
- T. Furukawa, Y. Hirakawa, H. Kondo, T. Kanemura and E. Wakai, “Chemical Reaction of Lithium with Room Temperature Atmosphere of Various Humidities,” Fusion Engineering and Design, pp. 2138-2141, 2014.
- “Drierite Desiccants,” [Online]. Available: https://secure. drierite.com/catalog3/page4b.cfm.
Acknowledgement
This paper was written by Gray Slough, Ph. D., Principal Applications Scientist at TA Instruments.
Click here to download the printable version of this application note.

