Keywords: MCAPN-2012-02, Microcalorimeters, Micelles, Nanospheres, Differential Scanning Calorimetry, Crystallinity
MC168
Introduction
The current role of liposomes, micelles and nanospheres include the ability to effectively deliver drugs to targets. Conventional liposomes or innovator molecules have been replaced with more advanced, modified liposomes. The size, composition, charge, and surface chemistry are varied to make a synthetically modified delivery vessel. These modifications are done in hopes of making the drugs they deliver more selective and thereby more effective.
Using DSC to Study Liposomes
Slowly heating a liposome in a Nano DSC instrument will result in an endothermic event as these molecules absorb heat at a particular temperature that is characteristic of the phospholipid composition of the liposome. This event is associated with non-covalent processes, including a conformational change in the packing of the acyl chains from trans to gauche.
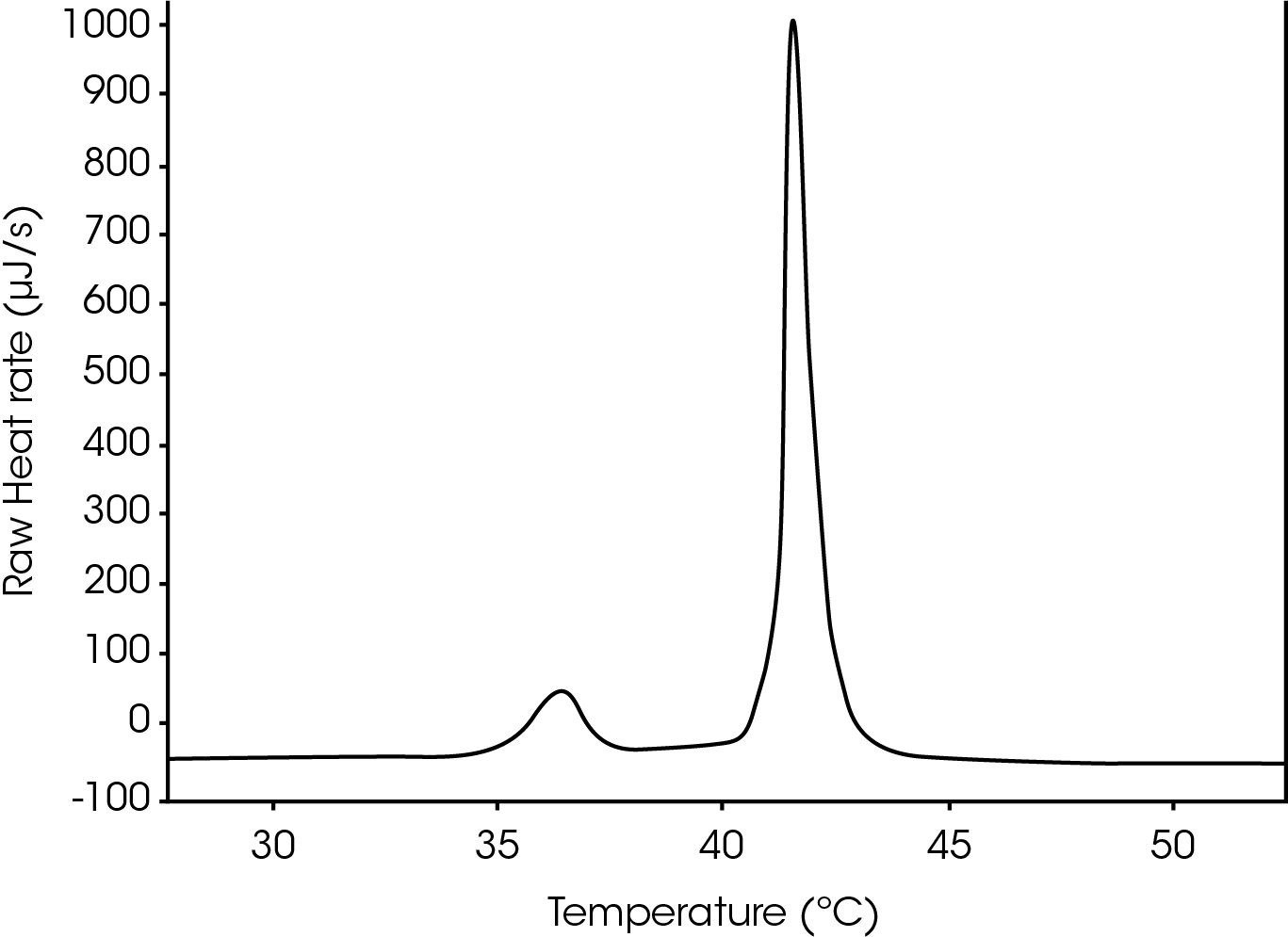
Before the heat induced transition, the alkyl chains are tightly packed in the gel phase, and after, they are less tightly packed in the liquid crystalline phase, a result of many of the side chains being in a gauche conformation. At the midpoint of the phase transition (Tm), vesicles in the liquid crystalline phase are increasingly dynamic and more permeable. Dipalmitoyl phosphatidyl choline (DPPC) is a liposome that serves as an excellent temperature standard, as well as a delivery vehicle. For the example DPPC, a pre-transition peak was also captured using the Nano DSC. This pre-transition phase has been identified as an event in which acyl groups become tilted. The Tm of the pre-transition was determined to be 36.4 ºC and the Tm of the main transition was 41.5 ºC, which were as expected when compared to literature values (Figure 1).
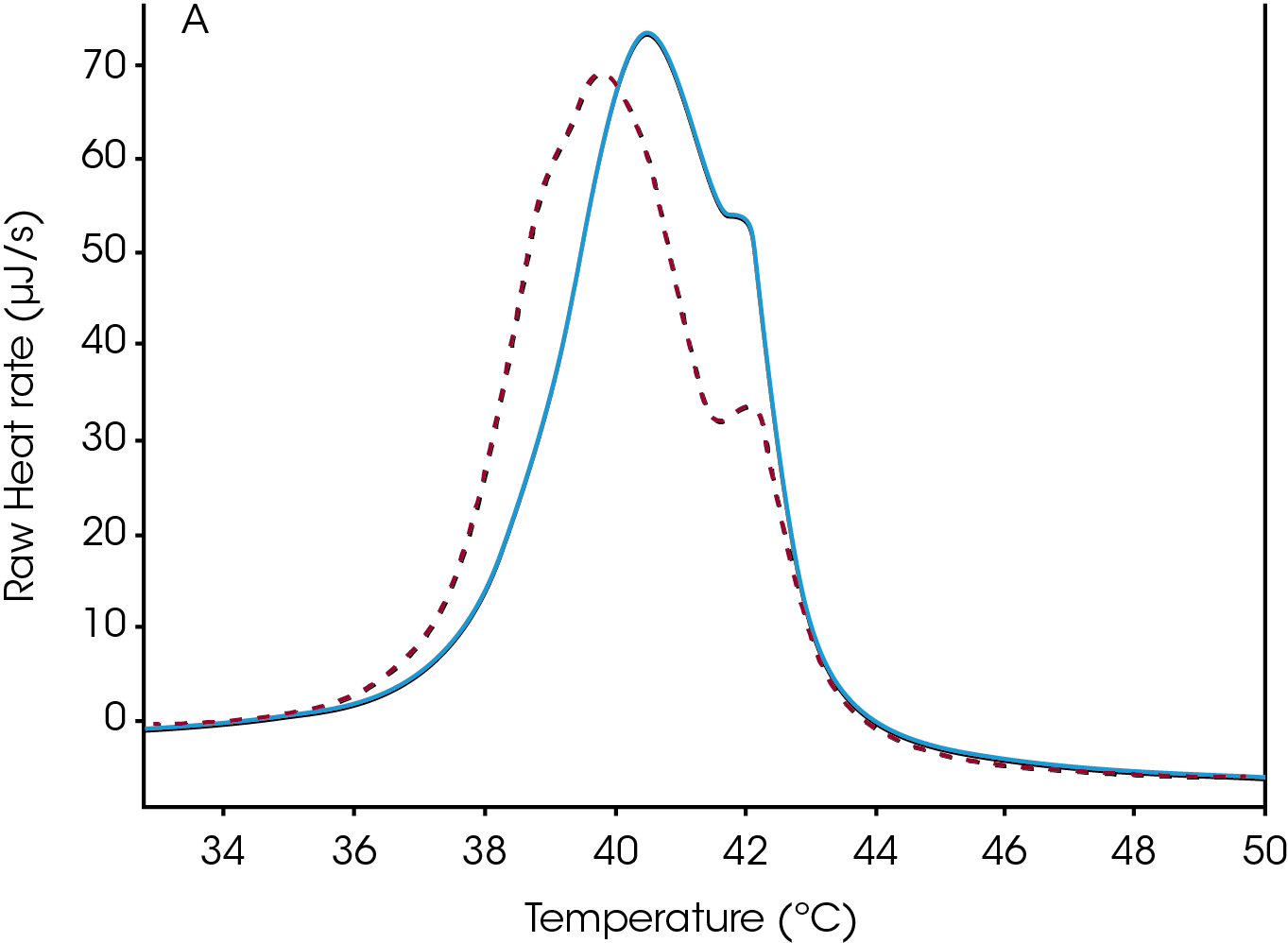
When considering data collection techniques in the Nano DSC, a further consideration must be made. It is often the case that when preparing these types of samples that the material is caught in a meta-stable state. The Tm of the prepared compounds is shifted after a heating and then reheating, which is typical for liposomes and like compounds that are prepared in a meta-stable state.
In the second example, a modified DPPC was used and the Tm shift is illustrated (Figure 2). The heating and then cooling allows these molecules to change from this meta-stable state to a more stable state and the Tm after the reheating is the most accurate value as after repeated reheating and cooling the Tm. There are other examples in which the first heating scan will yield no results while the second heating scan will reveal a transition.
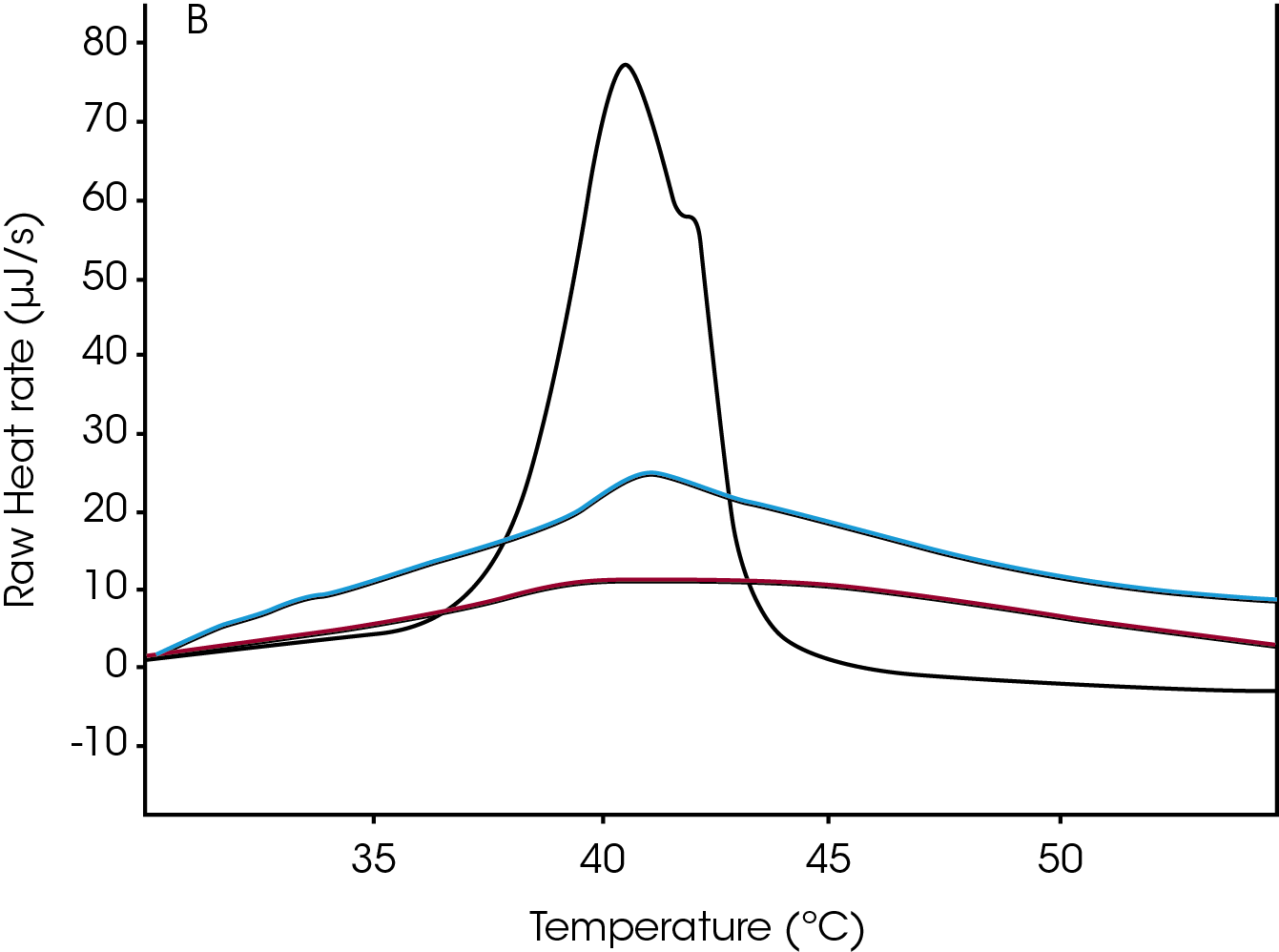
A second consideration in the thermodynamic analysis of liposomes is the accounting for molecular components added to modify the liposome. One additive that is commonly used because of its chemical properties and its importance in affecting membrane structure and integrity is cholesterol (Figure 3). Cholesterol increases the membrane/liposome fluidity; it prevents ordered packing of the acyl chains thereby increasing the fluidity and permeability of the membrane. At temperatures greater than the Tm, the rigid cholesterol molecule reduces the freedom of the acyl chains in the membrane and is less fluid than it would be without this small molecule. When a membrane is at a temperature lower than the Tm, it is normally in the gel phase, as mentioned above. The shift in the Tm was not significant for the samples containing cholesterol and this is most likely due to the steric constraints imposed by this small molecule.
The asymmetry of the profiles in the thermogram shown in Figure 3 indicates that this phase transition is not a two-state process. Also, the broad transition versus the tight transition gives insight into the process as one is slow and the other occurs more quickly over a tighter range. Broad transitions can also indicate impurities, but this may not be the case for these samples as these wide transitions are related to the properties of the material.
Summary
The Nano DSC instrument is amenable to studying liposomes. DPPC is a standard reagent routinely used to validate the temperature for fixed-cell DSC instruments. Preparation of the standard, as well as other liposomes, is not trivial. If liposome material is prepared with a method that result in a meta-stable state, obtaining a reproducible midpoint temperature of the major endothermic event will become highly dependent upon the reversibility of the thermal denaturation process. The sensitivity of the Nano DSC with baseline reproducibility at 28 nanowatts and short term noise at 15 nanowatts is a powerful tool for differentiating the thermodynamic properties of small amounts of material or the characterization of small signals and wide transitions.
References
- ASTM Standard E 2603, 2008, Standard Practice for Calibration of Fixed-Cell Differential Scanning Calorimeters. ASTM International, 100 Bar Harbor Drive, PO Box C700, West Conshohocken, PA (2008)
- Immordino, M., Dosio, F., and Cattell, L. Stealth liposomes: review of the basic science, rational, and clinical applications, existing and potential. International Journal of NanoMedicine.1. 297 (2006)
- Samsonov, A et al. Characterization of Cholesterol-Sphingomyelin Domains and their Dynamics in Bilayer Membrane. Biophysical Journal. 81. 1486 (2001)
- Simon K. and Ikonen E. How Cells Handle Cholesterol. Science. 290. 1709 (2000)
- Chiu, M. and Prenner, E. Differential Scanning Calorimetry: An invaluable tool for detailed thermodynamic characterization of macromolecules and their interactions. Journal of Pharmacy and BioAllied Sciences. 3(1). 39 (2011)
- Liposome cartoon: Lipids and Liposomes in the Enhancement of Health and Treatment of Disease” Young, S. and Smith, T.(2015)
Acknowledgement
This application note was written by Colette Quinn, Ph.D., Applications Scientist at TA Instruments.
Click here to download the printable version of this application note.

