Keywords: Drug Testing, Caking, Crystalline Material, TAM, Microcalorimeter
MC172
Introduction
It is well known that many water-soluble crystalline compounds after micronization have a poor physical stability when exposed to moisture. Problems with “caking” and severe aggregation of finely divided powders can be detrimental to the performance of the pharmaceutical product. It has been argued that micronization introduces amorphous regions, not measurable by X-ray diffraction, into the crystalline material. These amorphous regions transform, due to what appears to be surface sintering and recrystallization, at relative humidities well below the deliquesence point. This note reports the use of microcalorimetry as an analytical tool for characterizing micronized solids.
Experimental
The calorimetric experiments were performed using 2277 Thermal Activity Monitor equipped with the ampoule measuring cylinders. Disposable glass ampoules were used. A small plastic container with a saturated salt solution was placed inside the glass ampoule immediately before sealing it (see Figure 1). In this way the sample was maintained at a reproducible and well defined relative humidity all through the experiment. Furthermore, by varying the salt, different relative humidities were achieved. Experiments were performed with ‘micro-hygrostats’ containing saturated solutions of NaCl, KI, NaBr and Mg(NO3)2, which corresponds to relative humidities of 75%, 69%, 58% and 52%, respectively. All experiments were carried out at 25 °C.
Several batches of a micronized water-soluble drug compound were analysed. The crystalline drug compound has a deliquesence point at 97% relative humidity.
Results
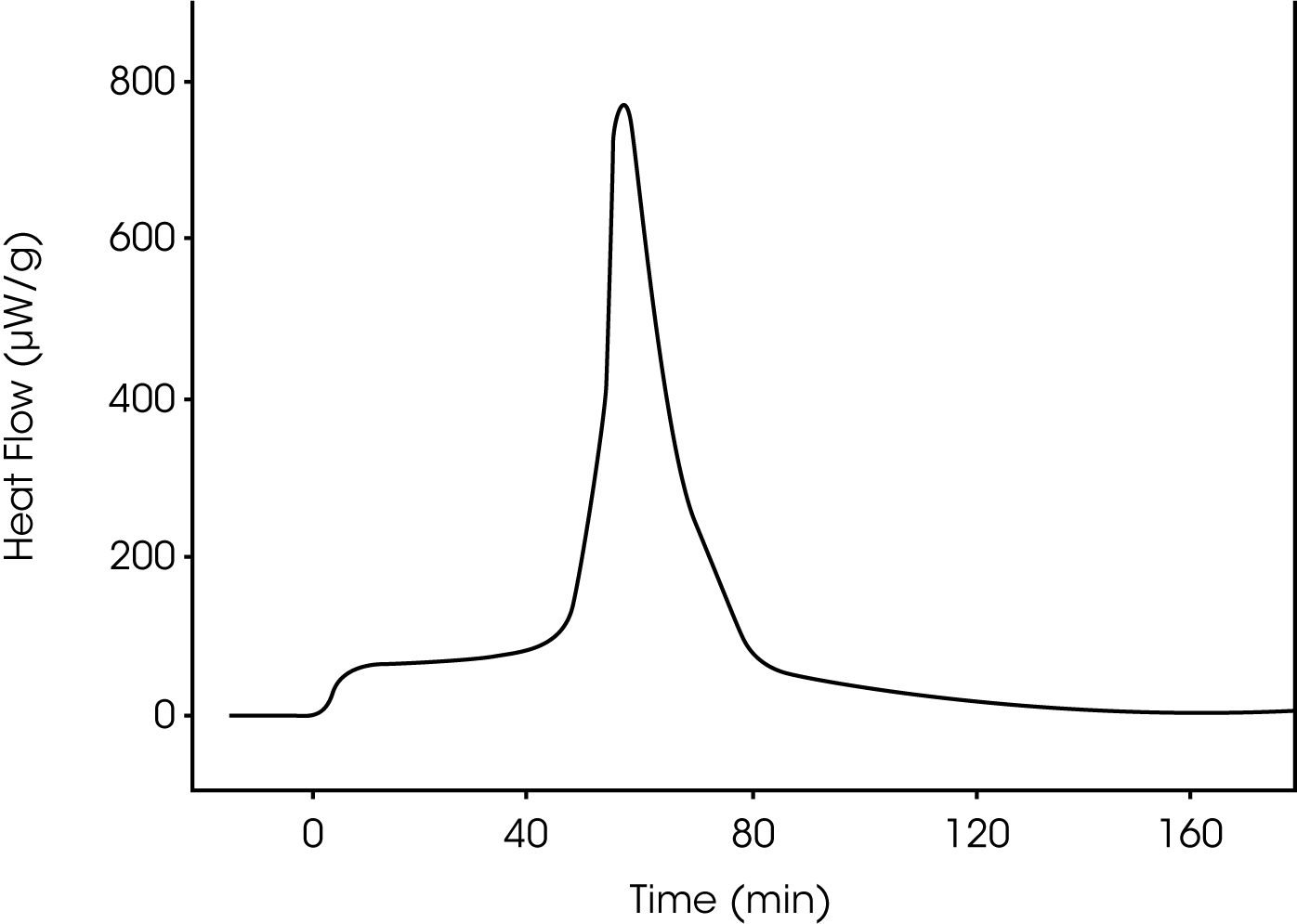
A typical heat flow curve is shown in Figure 2. When the micronized solid is exposed to moisture it produces heat at a constant rate for an initial period of 20-120 minutes (depending on experimental conditions). After the initial period heat is produced in a highly cooperative process resulting in a sharp peak in the heat flow curve. The calorimetric signal then returns to the baseline level.
The kinetics of the experiments agree well with the assumption of a moisture induced recrystallization. This “recrystallization” takes place in all the experiments independent of relative humidity. Furthermore, the cooperativity of the process is not influenced by the RH. The relative humidity does, however, affect the length of the initial period.
The total heat evolved during an experiment is surprisingly large and shows batch to batch variation. The value ranges from 3.7 to 7.0 J/g.
Some samples were analyzed by X-ray diffraction before and after the exposure to moisture. No changes in the diffraction signals were observed.
Conclusion
Microcalorimetry has proved to be an effective analytical technique for characterizing micronized compounds. It can be used to detect the presence of metastable regions not detectable by X-ray diffraction. Furthermore, the kinetics of “recrystallisation” of these regions can be studied, making a prediction of physical stability possible. Thus, the application of microcalorimetry in the pharmaceutical development has great potential.
Acknowledgement
Click here to download the printable version of this application note.

