Keywords: modulated DSC, epoxy, polymers, thermal analysis, TS-80, TS80
TA477
Introduction
Differential scanning calorimetry (DSC) measures the temperatures and heat flows associated with transitions in materials as a function of temperature or time in a controlled atmosphere. Modulated DSC (MDSC) is an enhancement to conventional DSC whereby the total heat flow is separated into reversing (heat capacity) and non-reversing (kinetic) components. A periodic temperature oscillation is utilized in MDSC, enabling the separation of these time dependent and time independent components [1]. The reversing signal contains heat capacity events such as the glass transition and melting. The non-reversing signal contains kinetic events such as crystallization, crystal perfection and reorganization, cure, and decomposition.
Experimental
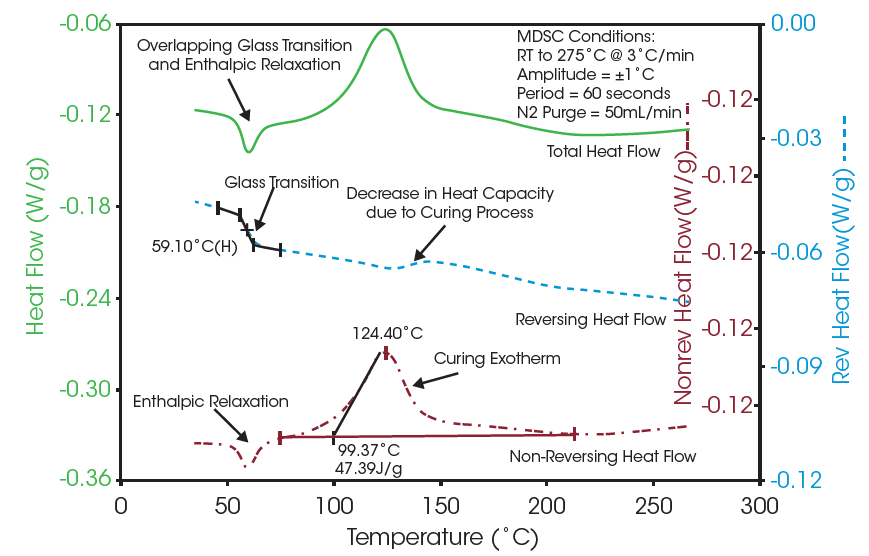
To demonstrate MDSC, an epoxy resin compound was analyzed using a TA InstrumentsTM differential scanning calorimeter. The sample was ramped from room temperature to 275 °C at a rate of 3 °C/min. An oscillation amplitude of ±1 °C and period of 60 seconds was used.
Results and Discussion
The above plot (Figure 1) shows an MDSC experiment on a sample of an epoxy resin compound. Analysis of the glass transition temperature and the curing exotherm using the total heat flow signal (conventional DSC) is difficult because of the overlapping enthalpic relaxation at the glass transition temperature and a decrease in heat capacity during the curing process. The MDSC data shows the glass transition temperature and overlapping enthalpic relaxation events clearly separated into the reversing and non-reversing heat flow signals. This decrease of heat capacity during the cure is vitrification. This is the point where the crosslinking networks are established. With this network established, the reaction essentially shuts down due to a decrease in molecular mobility and the reaction becomes a diffusion-controlled process. In addition, the decrease in the sample heat capacity, as expected during the curing process, is shown in the reversing heat flow signal. Analysis of these transitions by conventional DSC is difficult because the sum of all thermal events is shown in the total heat flow signal. Transition analysis is simplified by taking advantage of the ability of MDSC to separate heat capacity and kinetic related events into more easily analyzed signals.
References
- R.L. Danley, “TA322: New modulated DSC measurement technique,” TA Instruments, New Castle, DE, USA.
Acknowledgement
Click here to download the printable version of this application note.

