Keywords: Isothermal titration calorimetry, methanol, organic solvent systems
MC156
Abstract
Isothermal titration calorimetry (ITC) is a well-established tool to study binding of macromolecules. Understanding the non-covalent forces that drive these interactions can lead to meaningful advances in the fields of life sciences, pharmaceutical sciences, and drug development. However, the ITC has recently received increased attention from the field of material science, which necessitates the use of non-aqueous solvent systems. Herein, we elaborate on the development of a representative binding assay that measures the interactions of Ca2+ with a calcium ionophore in a methanol solvent system.
Introduction
Isothermal titration calorimetry (ITC) is a powerful analytical tool to study molecular binding. Its ability to make label free measurements was attractive to biochemists interested in measuring intermolecular interactions, catalysis, and binding equilibria. The study of biological systems involves a complex choreography of small molecule, non-covalent interactions. To this end, ITC is recognized as the industry standard for studying protein-small molecule binding, protein-nucleic acid binding, and protein-protein binding.1
All binding events either produce or consume heat, which corresponds to a change in enthalpy, ΔH. When these heat measurements are made at constant temperature, ITC can measure ΔH directly. As each successive injection is made throughout the course of the titration, all binding sites will eventually be occupied, allowing for the determination of the association constant, Ka.2 Temperature (T) is held constant, so the Gibbs energy can be calculated by:
ΔG = -RT ln Ka
The Gibbs energy equation can then be rearranged to provide the entropy of the reaction, ΔS:
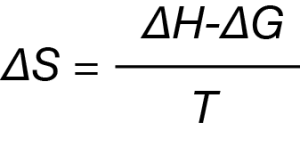
Taken together, ITC provides a full thermodynamic profile of the non-covalent interactions that drive binding events. The utility and ease of use of ITC in aqueous systems has raised the question of whether this technology can be harnessed to study binding in non-aqueous systems. Certainly, ITC could provide considerable benefits to those studying guest-host chemistry,3 protein and DNA chemistry in ionic liquids,4 and the behavior of engineered nanoparticles (ENPs).5 The purpose of this work is to highlight the ability of ITC to measure binding in organic solvents. Herein, we demonstrate the measurement of Ca2+ binding to a known calcium ionophore using an anhydrous methanol solvent system.
Experimental
Stock solutions of anhydrous CaCl2 (Millipore Sigma, CAS Number 10043-52-4) and Calcium ionophore II (Millipore Sigma, CAS Number 74267-27-9) were prepared at 10 mM and 1 mM, respectively, in MeOH that was dried using a molecular sieve. To account for evaporation, 400 µL of ionophore was loaded into the sample cell of a low volume gold semi-automatic Affinity ITC (TA|Waters) that was set to the Organic Mode. The titration profile involved 20 2-µL injections of Ca2+ spaced out by 200 s. A control experiment was performed that involved injecting 10 mM Ca2+ into dry methanol using the same experimental parameters as described previously. Data analysis was performed in NanoAnalyze v3.12.0 (TA|Waters) using the independent and blank (constant) models.
Results and Discussions
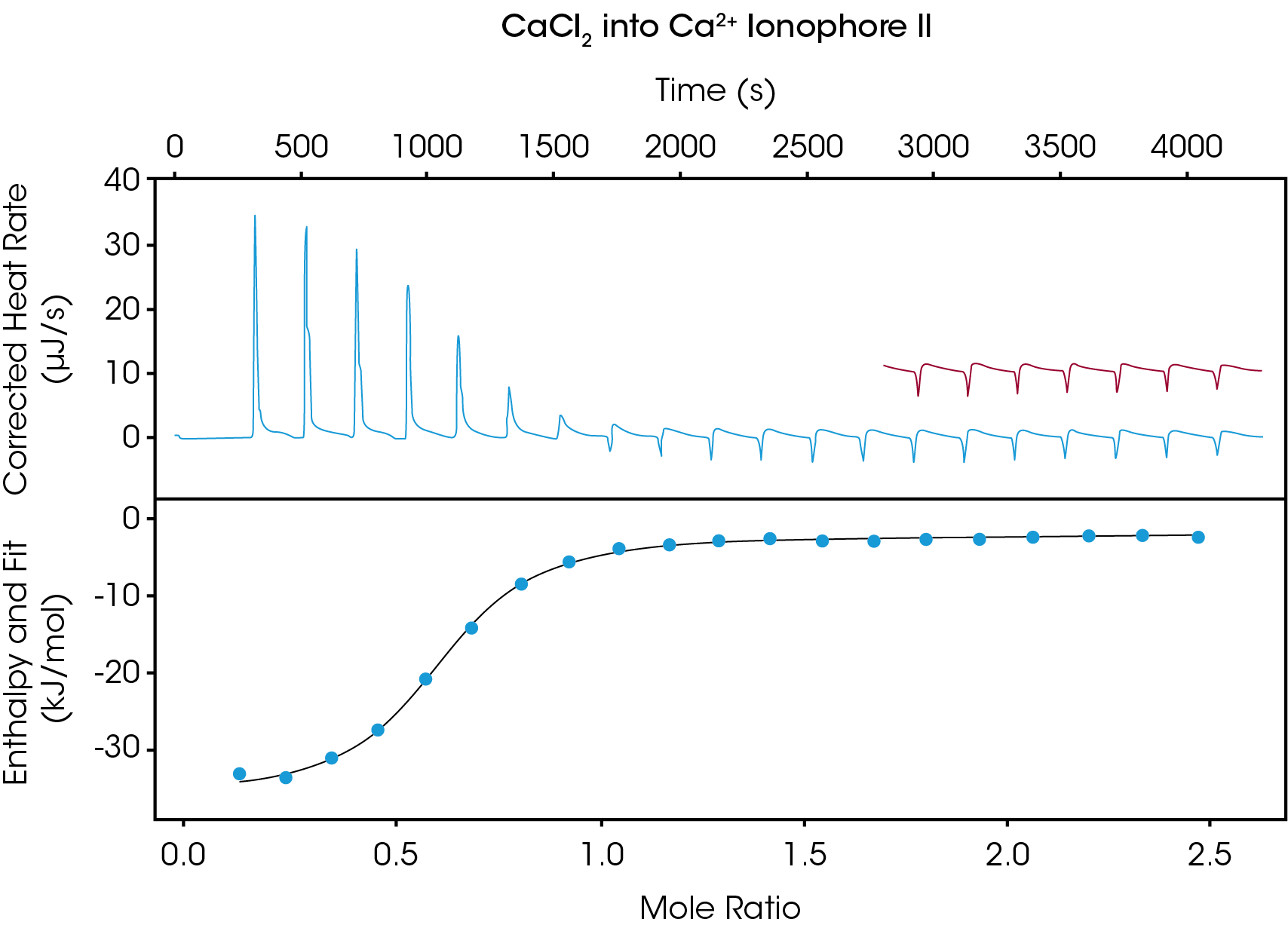
Figure 1 shows the thermogram obtained when performing the Ca2+ -ionophore titration. The top panel depicts the raw heat output in units of µJ/s, while the bottom panel displays enthalpy values of each injection as obtained by integrating the area under the injection peak. The injection spacing of 200 s was sufficient to allow the heat signal to return to baseline between injections, and ionophore saturation was achieved after approximately 10 injections. The remaining 10 injections were used to determine the blank constant value to subtract out during data analysis, allowing for a more accurate fit. Data analysis revealed a Kd of 24.05 µM, ΔH= -32.35 kJ/mol, and ΔS = -20.09 J/mol*K. The observed stoichiometry was observed to be n = 0.582, indicating that approximately two ionophores associate with each Ca2+ ion.
Conclusions
These results demonstrate the ability of the Affinity ITC to collect high quality binding data for systems under non-aqueous solvent conditions. The CaCl2 into Calcium ionophore II titration effectively represents the organic chemistry equivalent of the well-established CaCl2-EDTA titration that is used in the test kit. The goal of this work is to encourage continued method development with the Affinity ITC to address increasingly novel research projects.
Notes
When designing an experiment, be sure to confirm solvent compatibility with an Applications Scientist. Setting the instrument to the Organic Mode rather than Aqueous Mode will help to maintain instrument performance and longevity. Customers may also consider using the Nano ITC (TA|Waters), an instrument that was designed to accommodate a wade range of organic solvents. Please visit the application notes library on our website for more information about ITC.
References
- Vega, S. Abian, O. Velazuez-Campy, A. (2015) A unified framework based on the binding polynomial for characterizing biological systems by isothermal titration calorimetry. Methods. 76, 99-115.
- Wiseman, T.S. Williston, J.F. Brants and L.N. Lin (1989) Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal. Biochem. 179, 131-137.
- Garrido, P.F. Rodriguez-Dafonte, P. Garcia-Rio, L. Pineiro, A. (2021) Simple ApproximaTion for aggregation number determination by isothermal titration calorimetry: STAND-ITC. Landmuir. 37(40), 11781-11792.
- Shukla, S.K. Mikkola, J.-P. (2020) Use of Ionic Liquids in Protein and DNA Chemistry. Front. Chem. 8, 598662.
- Loosli, F. Vitorazi, L. Berret, J.-F. Stoll S. (2015) Isothermal titration calorimetry as a powerful tool to quantify and better understand agglomeration mechanisms during interaction processes between TiO2 nanoparticles and humic acids. Environ. Sci. Nano. 2, 541-550.
Acknowledgement
This paper was written by Dr. Samuel Redstone, Applications Support Scientist at TA Instruments.
Click here to download the printable version of this application note.

