Keywords: thermoset, Discovery X3 DSC, curing, epoxy, rubber
TA437
Background
The Discovery X3 Differential Scanning Calorimeter
The Discovery X3 Differential Scanning Calorimeter features a multi-sample cell which can analyze up to three samples simultaneously. This arrangement enables a significant boost in productivity and sample throughput. Thermosetting materials are routinely analyzed using DSC instruments to study curing and other thermal properties. The following paper discusses the advantages of the Discovery X3 DSC for thermoset analysis including the ability to study varied material compositions and curing conditions. Data can also be improved through replicate testing and statistical analysis, a distinct advantage of the Discovery X3 multi-sample cell.
Thermoset Material Analysis
Thermoset materials cross-link to form a cured structure, and DSC is used to measure this process. DSC can be used to measure the cure exotherm of a cross-linking reaction, as well as the resulting shift in glass transition temperature (Tg) due to cross-linking. A typical thermoset curing experiment using a DSC would be a heat-cool-heat experiment, where the first heat is run to the curing temperature to measure the exothermic curing peak. The second heat measures the Tg of the newly cured material, as well as any residual cure at the curing temperature.
Experimental
Two-Part Epoxy Curing Analysis
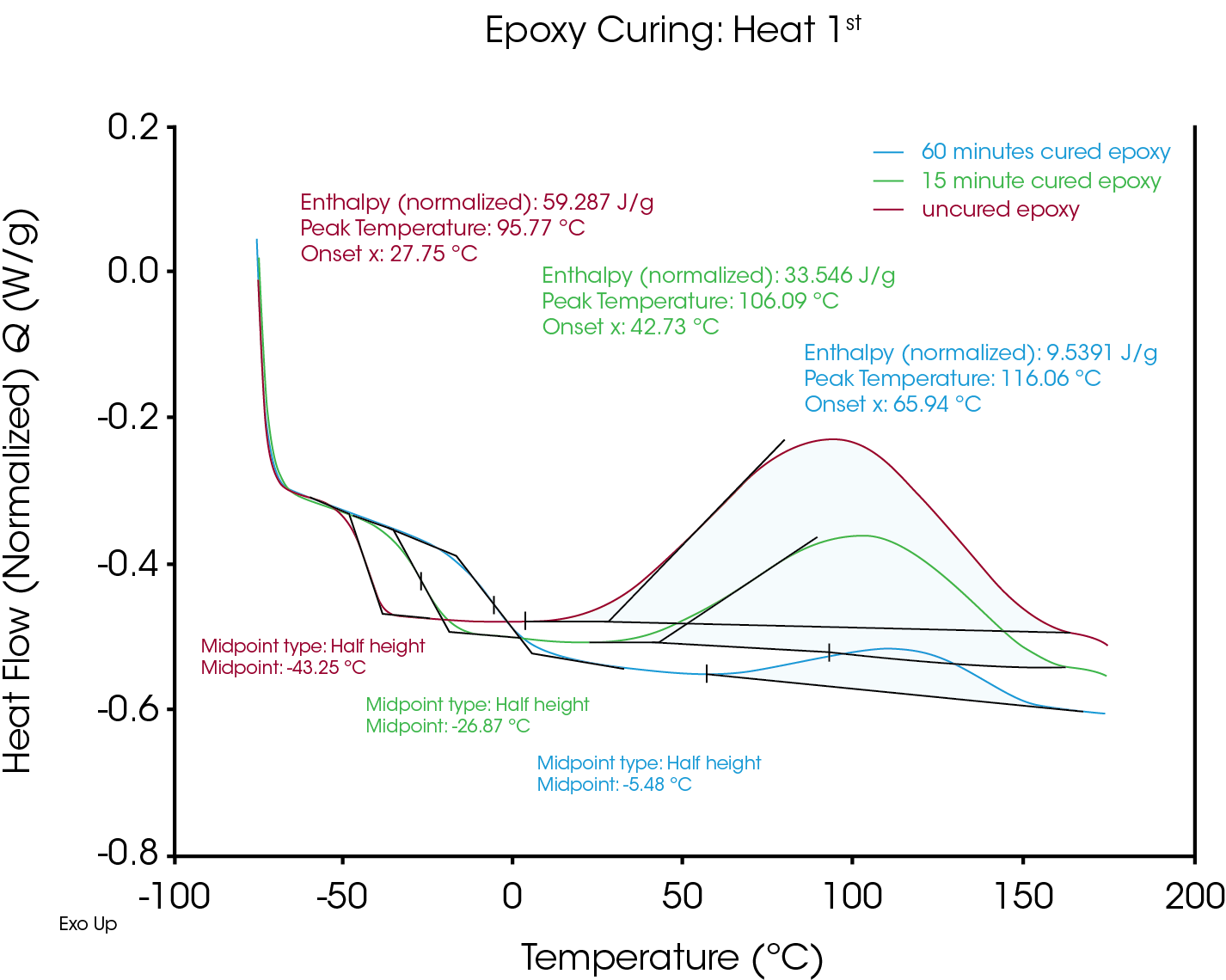
Figure 1 shows DSC data from the first heat of a two-part epoxy material. The packaging indicates the epoxy is set after 15 minutes, and “cured” after 30 minutes. Three samples were analyzed simultaneously using the Discovery X3 DSC; one that had been curing for 60 minutes (2x the manufacturers stated curing time), one that had been curing for 15 minutes (the set time), and one that was essentially uncured (placed in the DSC immediately after mixing).
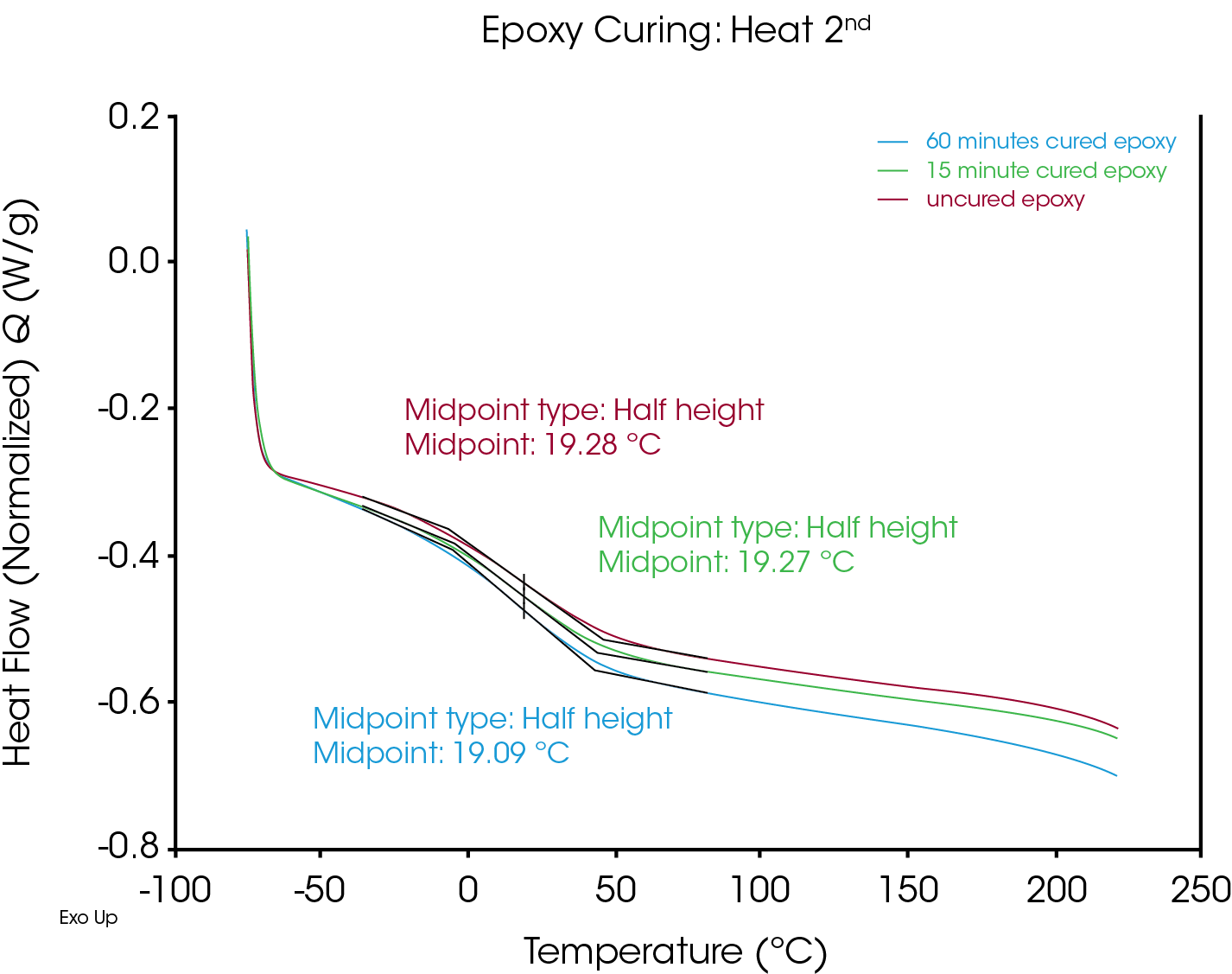
Using conventional DSC, all three samples would need to be separately mixed and run at the appropriate cure time. With the Discovery X3 DSC, time is saved by preparing each sample for the same instrument run and not needing to wait for individual runs to complete before preparing the next sample. This enabled 3 curing times to be quickly analyzed. The data shows expected trends in the size of each residual cure peak and the relative temperature and scale of the Tgs. As expected, the Tg of the fully cured products all occur at the same temperature in the second heat segment (Figure 2).
Despite the manufacturer’s recommendation, the 60 minute cured specimen still shows a visible exotherm. This information would be useful to an analyst studying this epoxy blend, and it would be generally useful for the following types of analyses:
- Failure analysis- thermoset failure is often associated with non-uniform curing or under-curing.
- Investigation of a new mixture- understanding the optimal curing conditions for a new mixture or composition by quickly screening multiple curing times and the associated residual cures.
- Screening a variety of new compositions simultaneously- the same curing experiment can be run on three distinct new compositions.
- Lot to lot variability and quality control- routine analysis is made easier and faster with the ability to run three samples in the same DSC experiment.
- Statistical analysis- samples run in triplicate using the Discovery X3 DSC all have the same thermal history, which enables statistical analysis of thermal transitions.
Peroxide Cure System Investigation
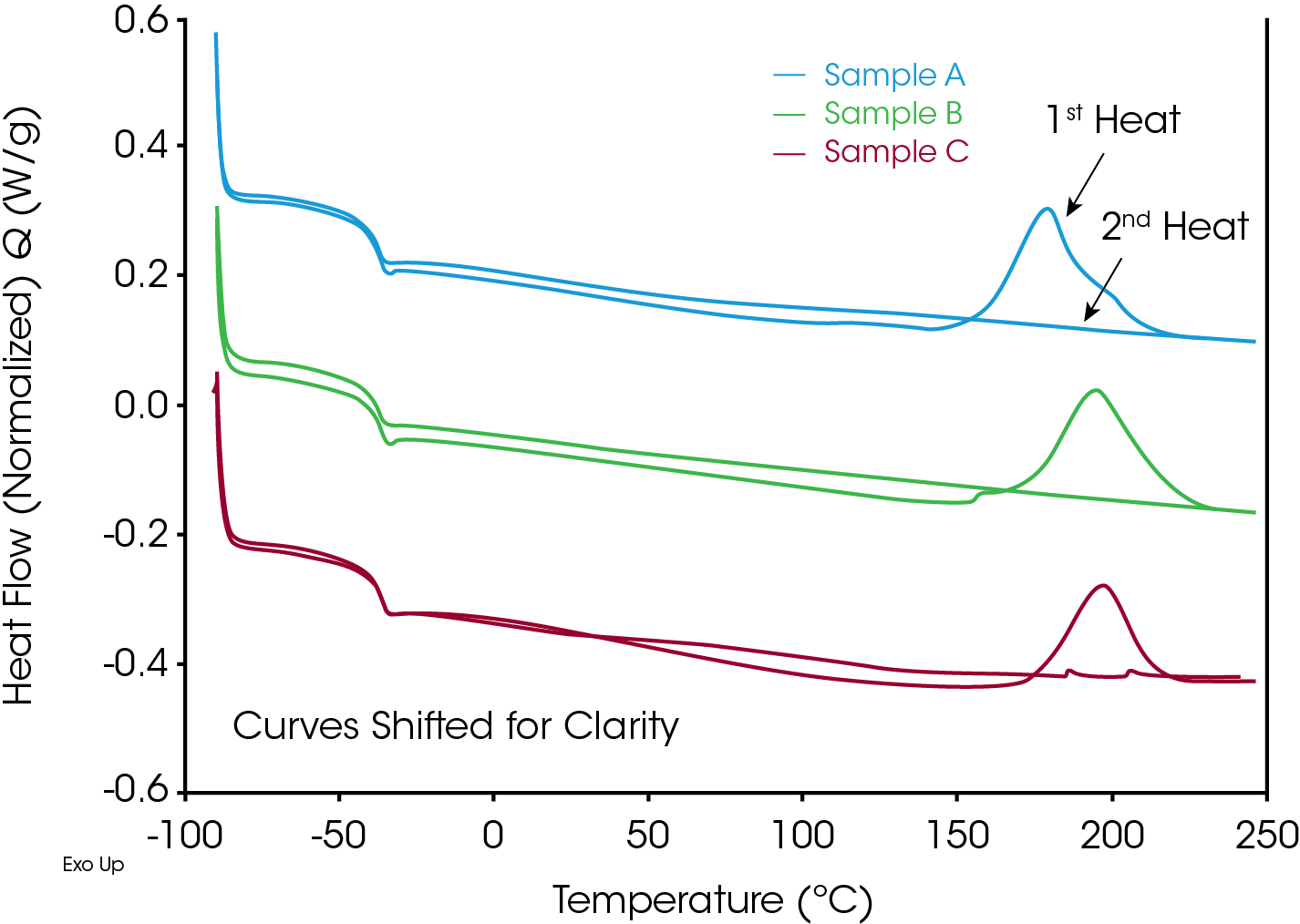
Three rubber samples were supplied by Parker Hannifin Corporation, each containing a different curing peroxide and designated samples A, B and C. A standard heat-cool-heat experiment was done to capture information about the cure exotherm and the Tg of these samples. Two types of heat-cool-heat experiments were performed on the Discovery X3 DSC; running all three samples at the same time, and running triplicates of each sample to obtain sample statistics.
Multiple insights about these three samples were gathered using the data from Figure 3. The data shows that Sample A cures at a lower temperature than Samples B and C, while Samples B and C appear to cure at about the same temperature. The second heat shows that each sample was fully cured during the first heat segment. Finally, it is observed that the Tg does not appear to shift between the 1st and 2nd heat, which is not a typical result.
Performing DSC experiments in triplicate is a natural extension of the Discovery X3 DSC. Figure 4 shows this experiment for rubber Sample A. There are two main sources of error in a DSC experiment- instrumental and from the sample itself. The temperature repeatability of the Discovery X3 DSC on an Indium standard is measured in the hundredths of a degree, while the enthalpy variation of an Indium standard is below 0.1%. So while the temperature and enthalpy differences in Figure 4 are not particularly large, they still remain outside the bounds of instrumental error and likely reflect real differences in the samples due to material variability or sample preparation. Running every sample in triplicate is usually not practical on a conventional single-cell DSC due to the instrument running time required. The Discovery X3 DSC enables triplicate testing without this impracticality. While the standard deviations reported in Figure 4 are low, one can envision scenarios where triplicate measurements and the resulting statistics would be advantageous such as:
- Sample heterogeneity- variation in the measured thermal events may reflect real differences in the material. This could be due to heterogeneity like incomplete mixing or other compositional differences when a small (typically <10 mg) sample is prepared for DSC.
- Improved data accuracy- reporting average values from triplicate testing results in more accurate and representative data.
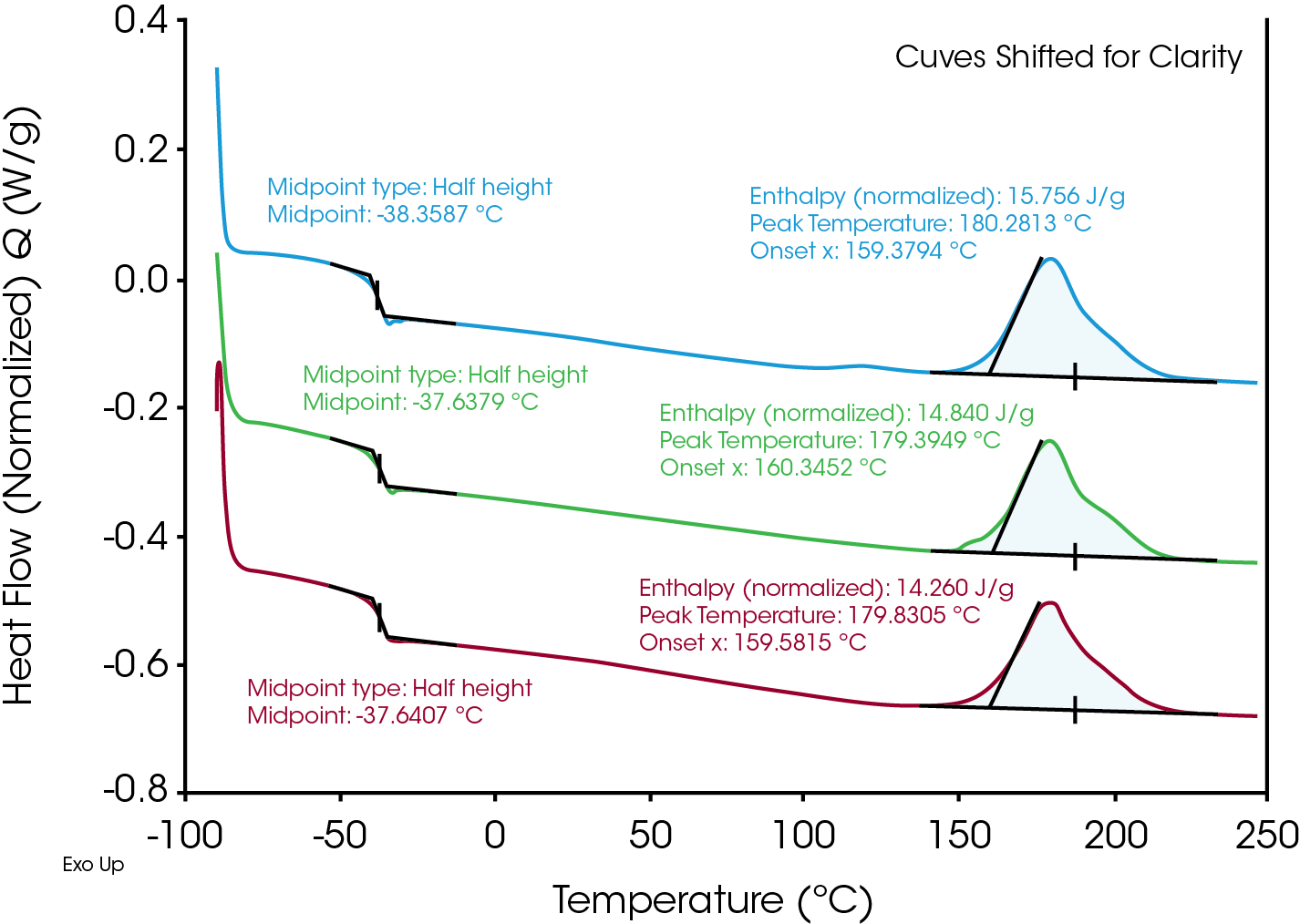
| Rubber Sample A | Tg (°C) | Enthalpy (J/g) | Exotherm Onset (°C) |
|---|---|---|---|
| Stage 1 | -38.359 | 15.756 | 159.379 |
| Stage 2 | -37.638 | 14.840 | 160.345 |
| Stage 3 | -37.641 | 14.260 | 159.582 |
| Average | -37.879 | 14.952 | 159.769 |
| Std. Dev. | 0.415 | 0.754 | 0.509 |
Conclusion
The experiments discussed here are representations of how DSC is generally used to analyze thermosets. It was shown that the Discovery X3 DSC presents many advantages over conventional single-cell DSC, even beyond the most obvious advantage of increased sample throughput. In addition to running existing samples three times faster, the Discovery X3 DSC makes it practical to collect three times as much data on those samples in the same amount of time. Techniques like collecting sample statistics through replicate testing is enabled when instrument throughput is increased threefold using the X3 DSC. This increased throughput is useful for a range of experiments such as screening more sample compositions and curing conditions in an R&D setting, or speeding up material comparisons in a production or quality control setting.
Acknowledgement
TA Instruments would like to thank Parker Hannifin for help in securing rubber samples for analysis.
Click here to download the printable version of this application note.

