Keywords: Oxidative Stability, Polyethylene
TA123
Summary
Perhaps no fundamental property affects the physical properties of a polymer in so general a way as the degree of crystallinity. Differential scanning calorimetry (DSC) provides a rapid method for determining polymer crystallinity based on the heat required to melt the polymer.
Introduction
An understanding of the degree of crystallinity for a polymer is important since crystallinity affects physical properties such as storage modulus, permeability, density, and melting point. While most of these manifestations of crystallinity can be measured, a direct measure of degree of crystallinity provides a fundamental property from which these other physical properties can be predicted.
Differential scanning calorimetry (DSC) is a technique which measures heat flow into or out of a material as a function of time or temperature. Polymer crystallinity can be determined with DSC by quantifying the heat associated with melting (fusion) of the polymer. This heat is reported as % crystallinity by ratioing against the heat of fusion for a 100% crystalline sample of the same material, or more commonly by ratioing against a polymer of known crystallinity to obtain relative values.
Experimental
In DSC, the sample contained in a metal pan and the reference (usually an empty pan) sit on raised platforms on the cell’s thermoelectric disk. As heat is transferred through the disk, the differential heat flow to the sample and reference is monitored by area thermocouples. A sample thermocouple directly monitors sample temperature. A preheated purge gas is present to provide additional baseline stability as well as the desired sample – atmosphere interaction.
In this study, samples of polyethylene were analyzed over the temperature range ambient to 180˚C. The programmed heating rate was 5˚C/minute; the atmosphere around the sample was nitrogen. Since the previous thermal history of a polymer affects the measured degree of crystallinity, these samples were evaluated both “as received” and after being subjected to a thermal treatment designed to impart equivalent thermal history to all three samples. This thermal treatment consisted of heating at 10˚C/minute to 180˚C, followed by controlled cooling at 5˚C/minute to ambient.
Results
Figure 1 shows the melting endotherm for one of the polyethylene samples during the initial “as received” heating. DSC Standard Data Analysis software was used to calculate the % crystallinity based on 290 J/g for a 100% crystalline material (1- 6). The results for the three samples studied are summarized below in Table 1.
These results clearly indicate that samples 1 and 2 are identical in terms of crystallinity and melt profile, suggesting that these two polymers had been previously subjected to identical processing conditions (thermal history). Sample 3, on the other hand, has a sharper melt and lower crystallinity indicating different processing conditions and different end-use properties.
After thermal treatment, the three polymers exhibit different crystallinities than initially obtained. The results are shown in Table 2.
These results reflect elimination of earlier processing thermal history effects. It is reasonable to assume that all these polymers would now have similar final properties. By subjecting polymer samples to different “thermal treatments” in the DSC prior to the crystallinity determination, it is possible to learn a lot about optimizing processing conditions.
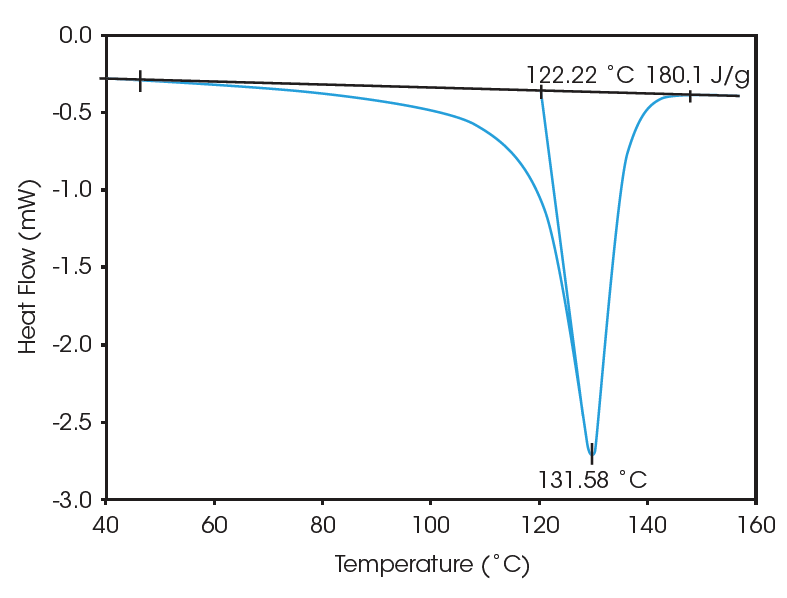
Table 1. “As Received” DSC Characterization of Polyethylene Samples
| Sample | Melt Onset Temperature (°C) | Melt Peak Temperature (°C) | Enthalpy (J/g) | Crystallinity (%) |
|---|---|---|---|---|
| 1 | 121.9 | 132.9 | 195.9 | 67.6 |
| 2 | 121.3 | 132.6 | 194.5 | 67.1 |
| 3 | 122.3 | 131.6 | 180.1 | 62.1 |
Table 2. DSC Characterization of Polyethylene Samples after a Common Thermal History
| Sample | Melt Onset Temperature (°C) | Melt Peak Temperature (°C) | Enthalpy (J/g) | Crystallinity (%) |
|---|---|---|---|---|
| 1 | 119.9 | 132.7 | 187.2 | 64.6 |
| 2 | 119.5 | 132.5 | 187.7 | 64.7 |
| 3 | 119.2 | 132.6 | 188.1 | 64.9 |
References
- M. Dole, W.P. Hettinger, Jr., N.R. Larson, and J.A. Wethington, Jr., J. Chem. Physics, 20, 781 (1952).
- B. Wunderlich and M. Dole, J. Polymer Sci., 24, 201 (1957).
- F.A. Quinn, Jr., and L. Mandelkern, J. Am. Chem. Soc., 80, 3178 (1958).
- B. Wunderlich and C.M. Cormier, J. Polymer Sci., Part A-2, 987 (1967).
- C.M.L. Atkinson and M.J. Richardson, Trans. Faraday Soc., 65, 1764 (1969).
- M.J. Richardson, J. Polymer Sci., Part C, 251 (1972).
Acknowledgement
This brief was developed by I. Groves. T. Lever, and N. Hawkins of TA Instruments, Ltd. (U.K).
Click here to download the printable version of this application note.

