Keywords: Isothermal Calorimetry, TAM Air, Cement Hydration, Geopolymer, Epoxy Curing, Metabolism, Soil Respiration, Biofuel
MC150
TAM Air
The TAM Air 8-channel calorimeter is a thermal activity monitor (TAM) with an air regulated thermostat. This 20 mL calorimeter holds up to 8 samples with a user-accessible matching reference. The differential design and latest electronics for control enable the 2 μW detection limit and a drift of less than 5 μW over 24 hours. The pinnacle of its performance is observed in stability of longer time periods, which is critical for the applications amenable to isothermal calorimetry.
Table 1. Performance Specifications TAM Air 8-channel (2019).
| TAM Air 8 Channel Calorimeter | |
|---|---|
| Temperature Range | 5-90 °C |
| Temperature Accuracy | ± 0.15 °C |
| Temperature Stability | ± 0.001 °C |
| Limit of Detectability | 2 µW |
| Short-term noise (5 min) | < 1 uW |
| Repeatability | 2 µW |
| Enthalpy Precision | 0.1% |
| Baseline Stability over 24 hr | |
| Drift | ≤ 5 µW |
| Standard Deviation | ≤ 2 µW |
| Error | < 6 µW |
Applications
Each section below is considered a major application field of use for the TAM Air. The publications and application notes listed are a starting point for further investigation into the literature.
Cement
Cement hydration has five different identifiable stages: heat of solution, dormant period, acceleration period, deceleration period with sulfate depletion, and steady period (1-3). When conducting studies on these complex mixtures with a closed ampoule, the final four steps are captured. An admix ampoule enables data acquisition of the initial heat of solution. Cement hydration is a practical application on the TAM, and it is accepted by the wider scientific community through the execution of ASTM Standards C1702, C1679, and European Standard EN 196-11.
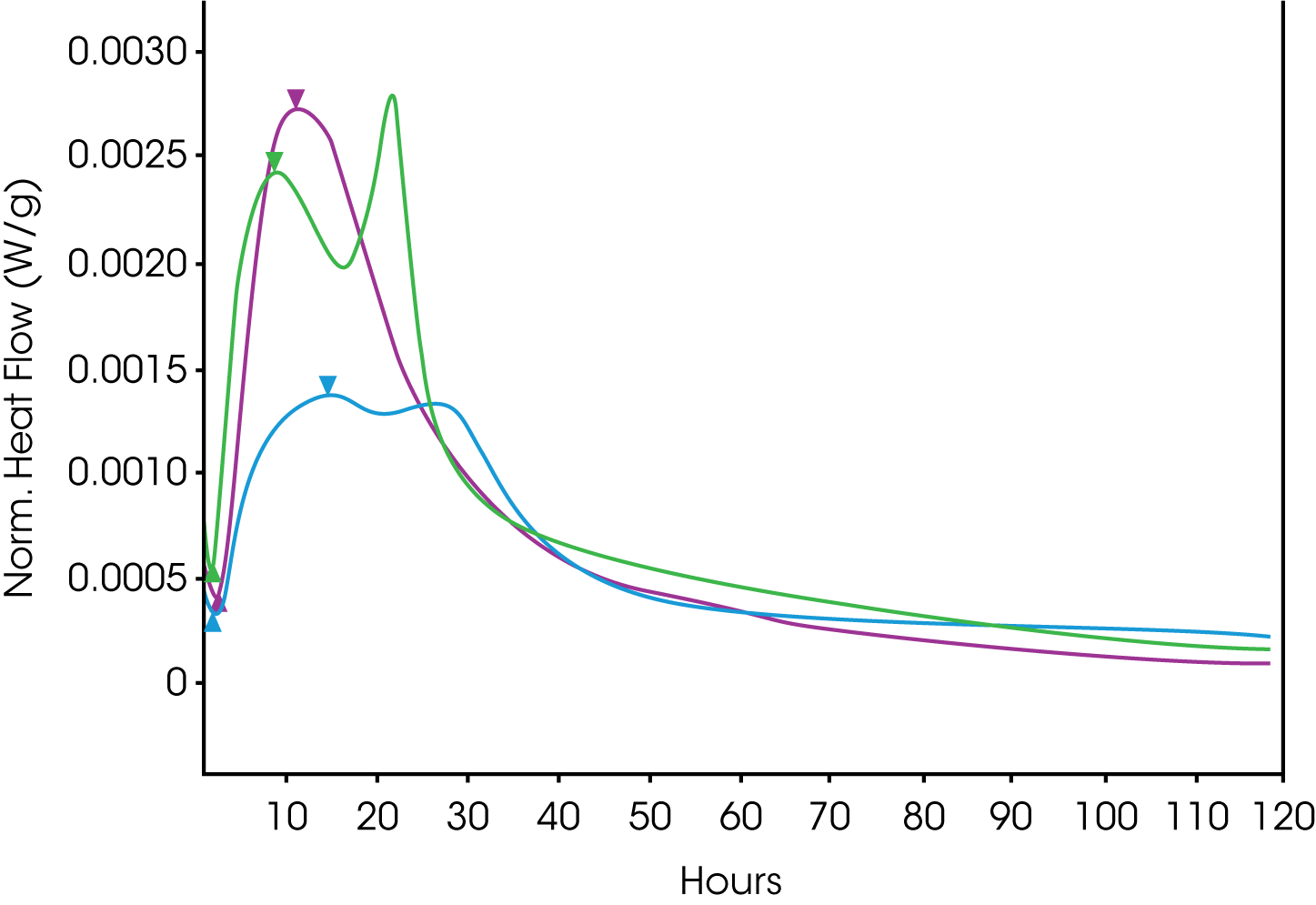
Geopolymers
Geopolymers can be used as an alternative to traditional ordinary Portland cement (OPC) as a cementitious building material. These relatively new polymers are commercially produced with specially tailored properties and the hydration process, like that of traditional cement, can be evaluated using the TAM Air. Some interesting isothermal studies include alkali mediated curing (4), investigation of reaction kinetics (5), and setting times effected by mechanical activation (6).
Metabolic Studies: Soil, Microbes, and Food
Metabolic experiments can be performed on the TAM Air, if the heat generated is greater than 2 μW (baseline repeatability). Calorimetry is a non-destructive technique and additional benefits include continuous real-time data and the capability of working with opaque or solid mediums (7). Examples of metabolic studies using the TAM Air include evaluating nutrient-enriched soils (8), food spoilage (7), and biomass conversion efficiency (9). Qualitatively, the faster the process occurs, the larger the amplitude of the heat flow. An admix ampoule can also be used to introduce nutrients or toxins while continuously monitoring the response. Extensive studies on metabolism and bacterial growth have been completed in the entire suite of isothermal calorimeters from TA by the Braissant group at the University of Basel, with an introduction to studying these processes in Reference 10.
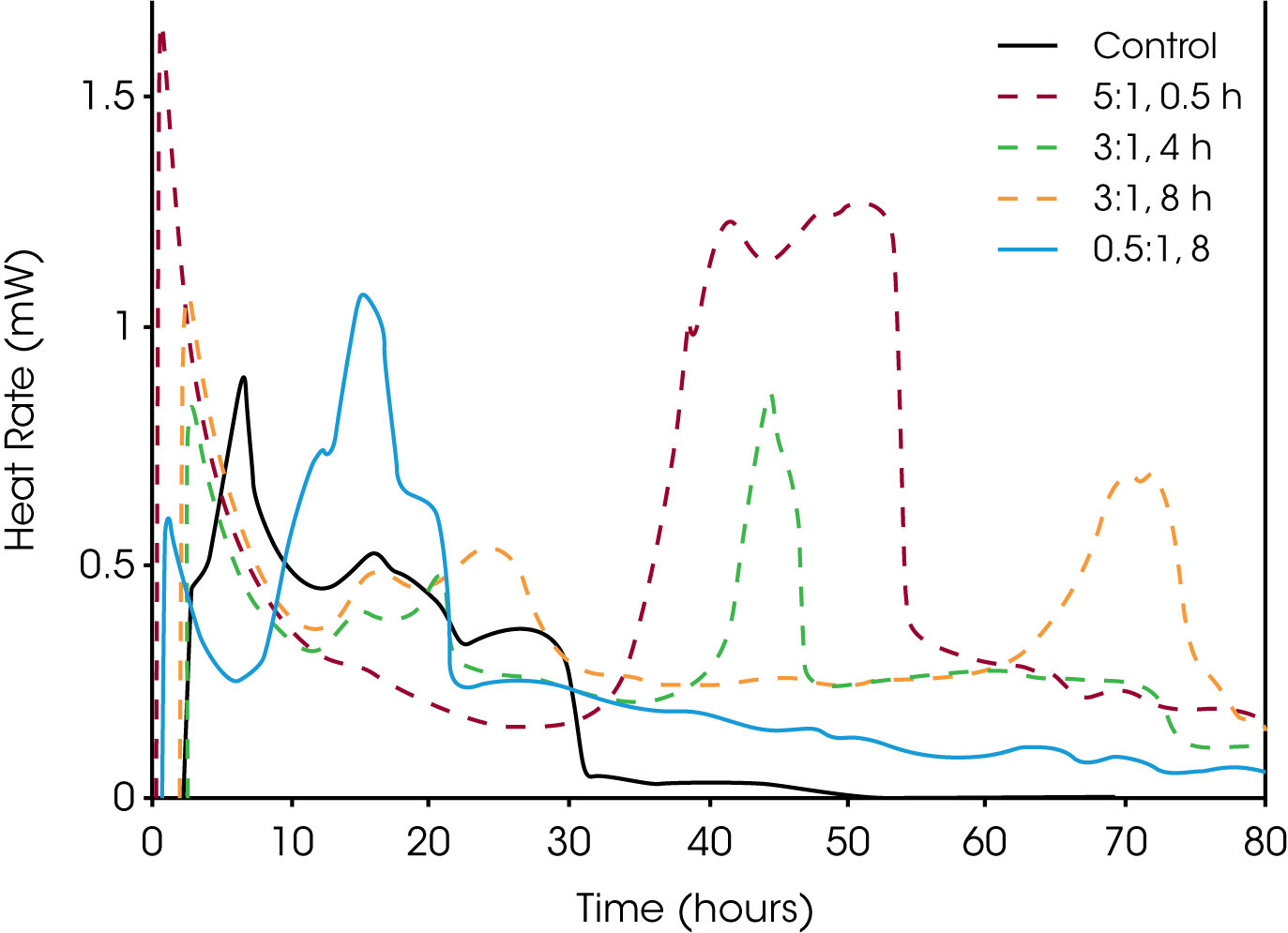
Solid State Biofuels
Biomass pellets are raw materials, commonly repurposed wood waste, compressed into pellets and used as fuel. These pellets are known to self-heat which can lead to a thermal runaway and ultimately spontaneous combustion; isothermal calorimetry can be used to predict these events (11,12). Studies and standards to better understand and control this process are currently underway. The international organization for standardization (ISO) is reviewing a standard to include isothermal calorimetry tests entitled: “Solid biofuels – Determination of self-heating of pelletized biofuels – Part 1: Isothermal calorimetry” (ISO/DIS 20049-1).
Epoxy and Biopolymer Curing
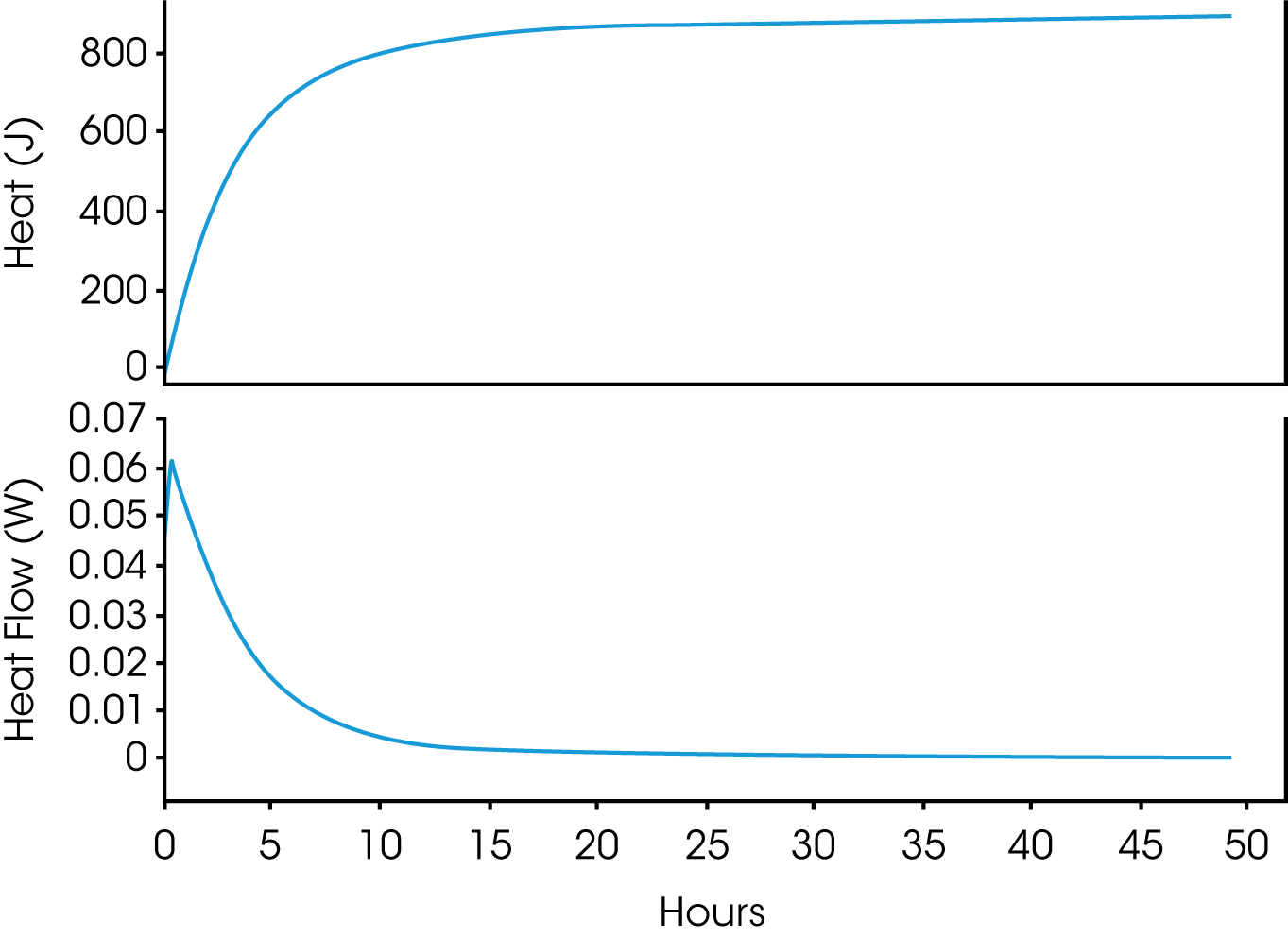
Thermosetting polymers are a growing class of compounds that encompass traditional epoxies to biopolymers (13,14). Formulation optimization can be studied by both differential scanning and isothermal calorimetry with each offering their own advantages (13, 14). They are complimentary in that one looks at temperature induced curing (DSC) and the other evaluates time and temperature dependent curing as well as residual curing (TAM Air). Plotting the reaction in terms of heat flow (W), shows how the reaction rate varies with time, and plotting as heat (J), shows how the extent of reaction varies with time. Further insight can be gained by varying the temperature to determine the reaction kinetics (15).
Conclusion
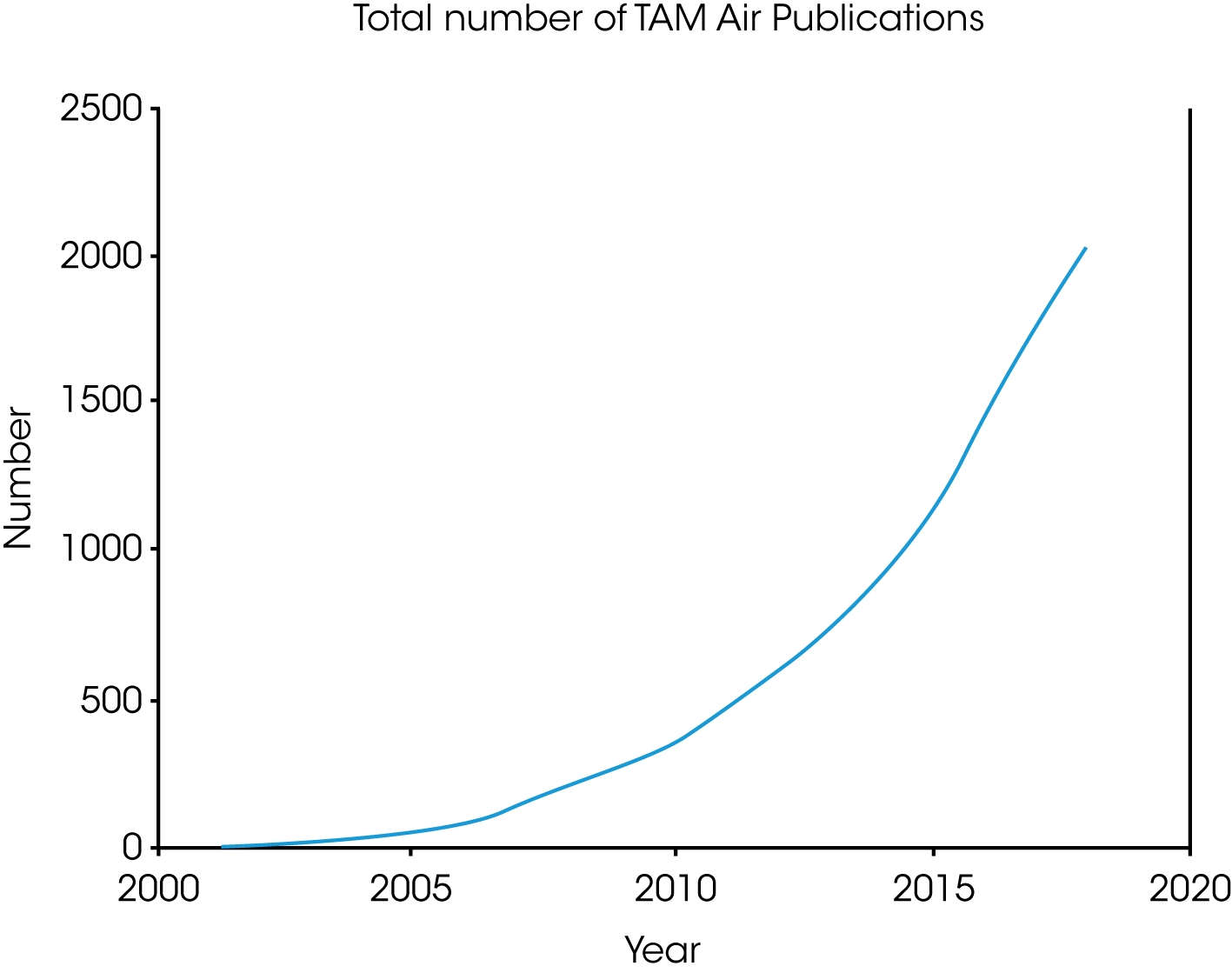
Increased use of isothermal calorimetry for solving product performance issues is shown by the exponential growth of TAM Air publications (Figure 6). The majority of the literature falls into the disciplines of materials, food, and ecology. However, because it is a non-destructive technique and the detection is universal in nature, increased usage is guaranteed in disciplines where product performance is linked to macroscopic and microscopic heat generated events.
References
- Wadsö, L. TA Application Note MCAPN-0100. “The Study of Cement Hydration by Isothermal Calorimetry”
- Sandberg, P. TA Application Note M594. “Optimization of Cement Sulfate Part II – Cement with admixture”
- Scrivener, K., Juilland, P., Monteiro, J. “Advances in understanding hydration of Portland cement” Cement and Concrete Research. 78. 2015 38-56.
- Yao, X. et al. “Geopolymerization process of alkali-metakaolinite characterized by Isothermal calorimetry” Thermochimica Acta (2009), 49–54.
- Nath, S.K. et al. “Kinetics study of geopolymerization of fly ash using isothermal conduction calorimetry” J Therm Anal Calorim. (2016), 1953-1961.
- Kumar, S., Kumar, R. “Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer” Ceramics International (2011) 533–541.
- Wadsö, L. and Galindo, F. “Isothermal calorimetry for biological application in food science and technology” Food Control (2009), 956-961.
- Quinn, C. TA Application Note MCAPN-2015-01. “Hot Holobionts! Using calorimetry to characterize these interactions”.
- Mathews, M. and Hansen, L. TA Application Note MCAPN-2012-04 “Following Anaerobic Digestion of Pretreated Algae by Calorimetry”.
- Braissant, O. et al. “Use of isothermal microcalorimetry to monitor microbial activities” FEMS Microbiology Letters (2010), 1-8.
- Larsson, et. al. “Development of screening test based on isothermal calorimetry for determination of self-heating potential of biomass pellets” Fire and Materials (2017) 940-952.
- Guo, W. et al. “Measurements of wood pellets self-heating kinetics parameters using isothermal calorimetry” Biomass and Bioenergy (2014), 1-9.
- McCoy, J. et al. “Cure mechanism of diglycidyl ether of bisphenol A (DGEBA) epoxy with diethanolamine” Polymer (2016), 243-254.
- Cuvellier, A. et al. “Selection of healing agents for vascular self-heating application” Polymer Testing (2017), 302-310.
- Lundgren, J., Hesse, N. TA Application Note MA002 “The use of isothermal microcalorimetry to characterize the cure kinetics of a thermoset epoxy material”.
Acknowledgement
This note was written by Colette Quinn, Ph.D., Microcalorimetry Product Manager (TA Instruments)
Click here to download the printable version of this application note.

