Keywords: Differential scanning calorimetry, pharmaceutical material, polymorphism
TA302
Abstract
Many pharmaceutical materials exhibit polymorphic behavior in which the substance may exist in several crystalline forms depending upon processing conditions. Differential Scanning Calorimetry (DSC) is a useful tool for detecting and quantifying these different forms.
Introduction
Many molecular crystals are capable of solidifying in multiple crystalline forms. The crystalline form of an active pharmaceutical ingredient impacts its physical properties and subsequent therapeutic performance. There are examples from the pharmaceutical industry where the appearance of a new crystal form significantly affected the performance of a product, sometimes with serious clinical effects. Important legal factors related to patent issues are also involved since the appearance of a new crystal structure is usually not covered by patent (1). For these reasons, new active ingredients are screened for polymorphic behavior to reveal the existence of multiple crystalline phases (2).
Dexamethasone acetate finds use as an anti-inflammatory agent. Anhydrous dexamethasone acetate is an example of a pharmaceutical material that exhibits polymorphism and is a useful tool for demonstrating the effects of different heating rates.
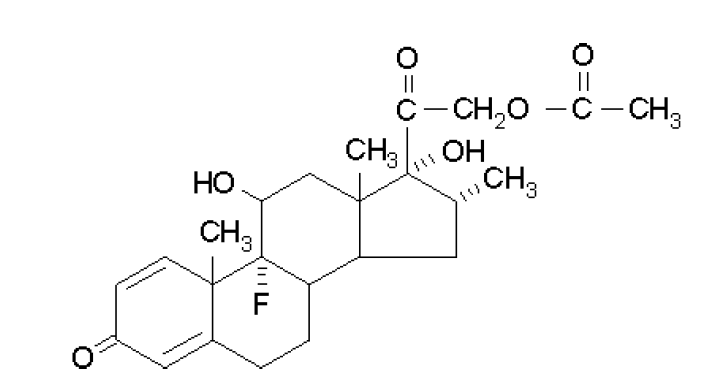
Experimental
Differential Scanning Calorimetry (DSC) measures temperature and enthalpy (heat) associated with transitions in materials. This technique provides quantitative and qualitative information about physical and chemical changes that involve endothermic or exothermic processes, or changes in heat capacity (3).
DSC analysis of anhydrous dexamethasone acetate is carried out using a TA Instruments Q-Series™ Q1000 DSC with the Refrigerated Cooling System (RCS). The sample is continuously purged with 50 mL/min nitrogen (99.999 %). About 1 mg of the pharmaceutical substance is crimped in an aluminum standard sample pan with lid. For each analysis, a new sample is prepared. First heating is accomplished using heating rates between 10 and 150 °C/min. To achieve the high heating rates of 100 and 150 °C/min the Heater PID Control method segment is used (4).
Results and Discussion
(Note: The thermal curves shown in Figures 2 and 3 are plotted with exothermic behavior in the downward direction, as is the convention in Germany. The choice of convention, exotherm upward or downward, is selectable in Universal Analysis software.)
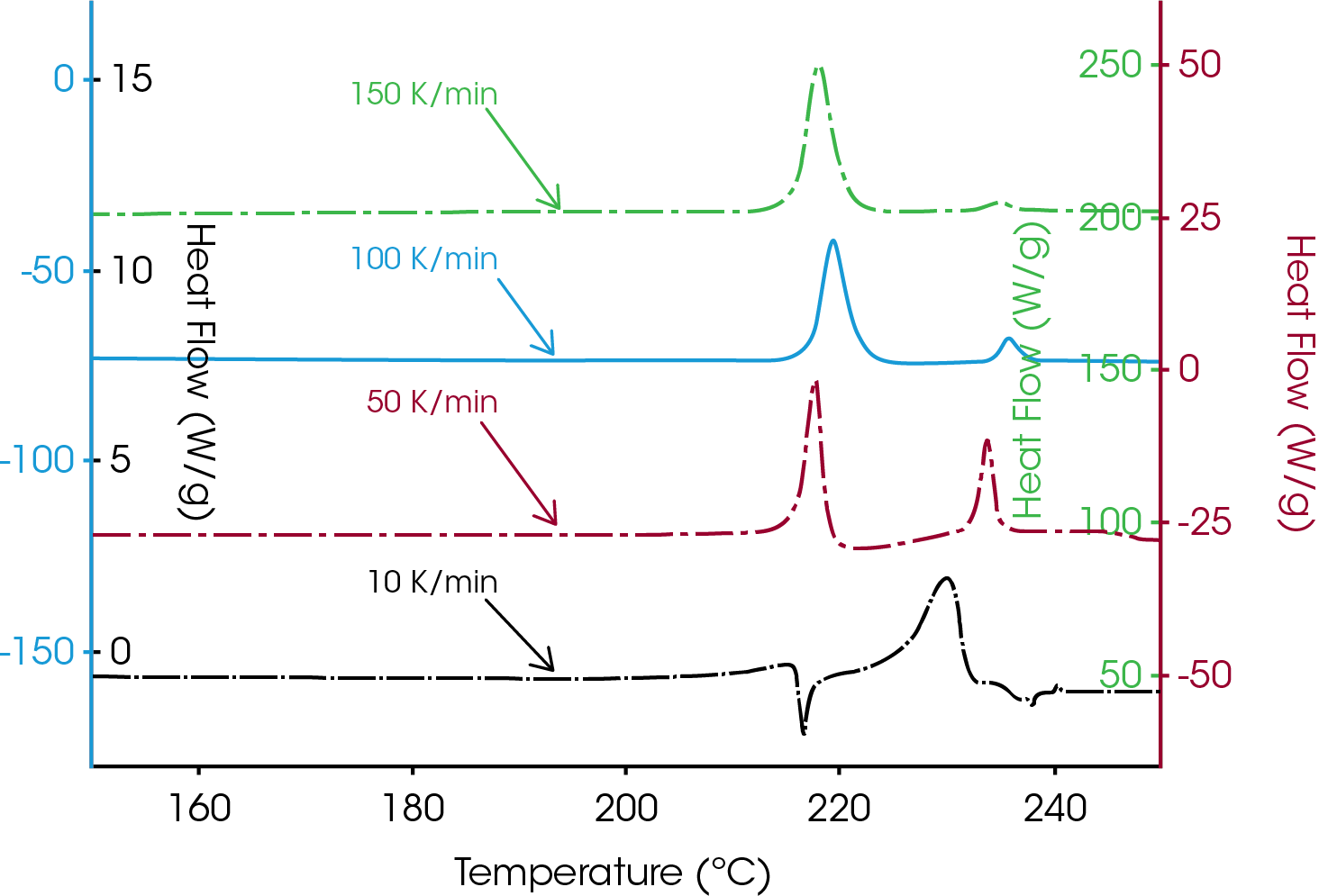
Figure 2 shows the comparison of the first heating of anhydrous dexamethasone acetate at four heating rates ranging from 10 to 150 °C/min. The samples exhibit two different endothermic transition crystalline forms melting between 210 and 240 °C. Different heating rates emphasize two polymorphism effects of the material:
- The peak maximum of both forms varies with the heating rate. The peak maximum of the first melting crystalline form (near 210 °C) decreases with decreasing heating rate, the second melting form shows a higher temperature in peak maximum with higher heating rates.
- The amount of the higher melting form increases with decreasing heating rate as indicated by peak area.
The analysis at commonly used 10 °C/min shows a small onset to melting around 210 °C at the beginning of the first melting. This is followed by recrystallization exotherm. The second melting form is created by the recrystallization of the first form during heating.
Above 235 °C the material begins to decompose. For that reason the melting is irreversible. With a heating rate of 1 °C/min, the decomposition starts before melting (no figure). But with the use of higher heating rate the decomposition is shifted to a higher temperature range. At 50 °C/min little crystallization is detected during heating. Both crystalline forms are melting. With increased heating rates (100 and 150 °C/min) the amount of the second crystalline forms decreases even more.
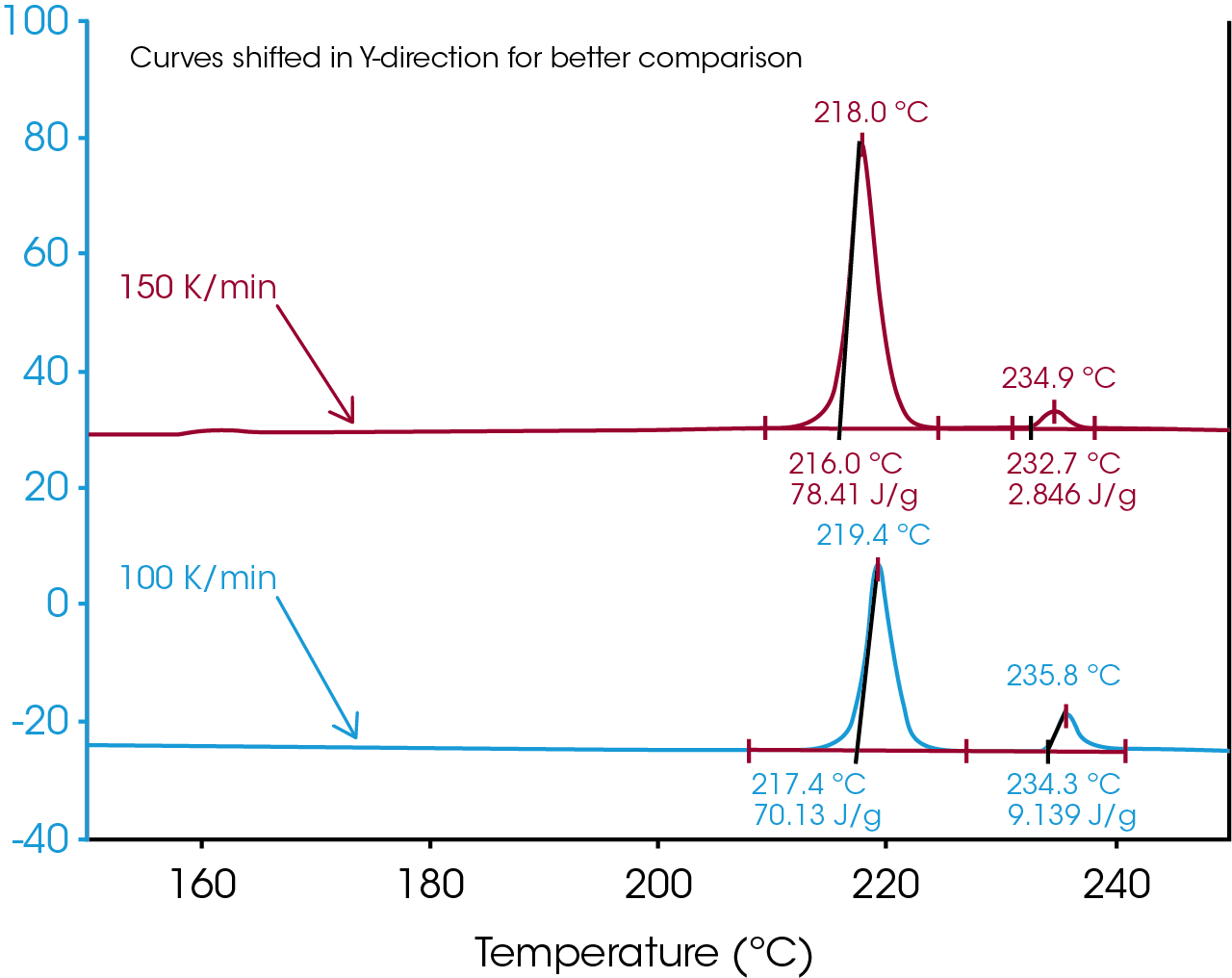
As result of high sensitivity, high resolution and a flat baseline the melting enthalpy of these two crystalline forms can be quantitatively analyzed even at heating rates in excess of 100 °C/min (Figure 3).
Conclusion
Differential Scanning Calorimetry is a useful technique for the characterization of polymorphism in pharmaceutical materials. For the detection of small polymorphic forms and separation of these forms an instrument with high sensitivity, high resolution and a very flat and stable baseline is needed. To exhibit the different crystalline states it is necessary to use high heating rates. The DSC Q-Series Q1000 DSC offers all these features to measure the different polymorphism of pharmaceuticals.
References
- N. Hall, “Predicting Polymorphism”, Pharmaceutical Formulation & Quality February/March 2000
- M. H. O`Neill and G. G. Sweetapple, “Polymorph Screening, Quantitation, and Identification of the Stable Phase”, 3rd International Symposium on Polymorphism and Crystallization, San Francisco, CA, November 12-13, 2001
- TS-54, “Characterization of the Degree of Cure of Thermosetting Resins by DSC”, TA Instruments, New Castle, DE.
- TN-47, “Heater PID Method Segment”, TA Instruments, New Castle, DE.
- U.S. Pharmacopoeia, United States Pharmacopoeia Convention, Rockville, MD, 1999, pp. 396-397.
Acknowledgement
Click here to download the printable version of this application note.

