Keywords: gasification, high pressure TGA, MS, coal , waste, steam, carbon dioxide
TA466
Introduction
Gasification is a sustainable technology to obtain syngas for the production of polymers and chemicals from solid fuel materials like coal, biomass or waste. Because gasification can be applied to a very broad variety of diverse feedstock materials with varying decomposition properties, it is desirable to test potential materials in order to identify the most efficient operating conditions (gasification agent, temperature and pressure). Using a High Pressure TGA instrument with the possibilities to apply a pressure range up to 80 bar and common gasification agents such as oxygen, air, CO2 and steam is a very convenient way to test feedstock. For the analysis of the product gases, online MS or spectroscopic methods such as FTIR are great tools to measure the gas composition as it changes with temperature, pressure or gasification agent concentration.
A lignite coal sample and several petroleum coke samples have been gasified in the new Discovery HP-TGA 75 and 7500 instruments using carbon dioxide and steam as gasifying agents. The evolved gas atmosphere was analyzed with online mass spectrometry to detect the generated syngas components.
Introduction
Gasification is a technology that has been widely used since the 19th century to produce gas for street lighting, as vehicle fuel or for other purposes. It has been an important fuel supply in countries facing a shortage of natural gas or crude oil. Today, gasification is an alternative to the use of conventional fossil resources to produce polymers or chemicals with a lower CO2 footprint using biomass or waste feedstock materials. Coal and petroleum coke are supplied into large gasification plants since gasification transforms the solid carbonaceous material into gaseous or liquid hydrocarbon feedstock for chemical processing. This enables recycling of the molecular building blocks of the materials in a sustainable economy.
More gasification plants are being constructed around the world to use the molecular content of coal and waste in a more effective way and to replace fossil fuels in the production of chemicals and polymers with biomass and waste materials in order to reduce CO2 emissions.
A gasification process consists of several steps including drying of the feedstock and pyrolysis followed by a high temperature reaction with a gasification agent which is usually CO2, air or steam.
In the presence of CO2, the carbon content of the material is reacting mainly to carbon monoxide following the Boudouard reaction C + CO2 ←→ 2 CO. When steam is used as gasification agent, hydrogen is generated as well, one of the major reactions is C + H20 ←→ CO + H2. Gasification plants are normally operated in a pressure range of 10-40 bar because the reaction is more energy efficient under these conditions.
Process temperatures usually range above 1000 °C, so that energy is an important cost factor. Especially when feedstock properties vary, optimization of the reaction conditions is important to operate the gasification plant in the most efficient way.
Useful insight for the operation of a gasification plant can be obtained from high pressure TGA instruments: the weight signal represents the conversion rate and shows how reaction conditions determine the gasification kinetics of various feedstock types [1-4]. Additional data can be obtained when the generated syngas is analyzed with subsequent analysis techniques such as online Mass Spectrometry, FTIR or Gas Chromatography [5].
At TA Instruments, two high pressure TGA instruments are available: the DynTHERM instruments based on the traditional Rubotherm style Magnetic Suspension Balance and the new Discovery HP-TGA product line of benchtop HP-TGA instruments [6,7]. DynTHERM instruments are capable of measuring with larger sample sizes and higher temperature ranges, Discovery HP-TGA instruments are benchtop instruments with a smaller footprint. The Discovery product line combines the ease of operation of a standard TGA with powerful features such as fast heating and cooling rates, high steam ratios and Curie point temperature calibration.


Experimental
For this study, two sample types where selected: a lignite coal powder sample with a high content of volatile matter and humidity and a set of four petroleum coke samples with varying ash content representing the typical waste material as is obtained in large amounts in crude oil refineries. Samples were analyzed by high-pressure TGA at various temperatures and pressures with CO2 and steam as gasification agents to study the influence of the reaction conditions on the conversion rate.
The high pressure TGA instruments used in this work were a Discovery HP-TGA 75 and 7500, the use of DynTHERM systems for gasification experiments is already well described in the literature [1-5]. The HP-TGA 75 allows to switch between three different reaction gases. To simulate typical gasification conditions, a gas-cylinder with a mixture of 30% CO2 in argon (Linde Gas) was used as reaction gas. The HP-TGA 7500 features a steam generator that was used for steam gasification measurements, both instruments have a temperature range up to 1100 °C and a pressure range up to 80 bar.
At elevated pressures, TGA analysis requires data correction, because a temperature gradient applied to a compressed gas atmosphere changes the density of this reaction atmosphere significantly. When the sample weight is measured under such conditions, the weight signal is overlayed by the buoyancy effect. In high pressure TGA, this discrimination can be corrected by subtracting a blank run. The Discovery Series HP-TGA instruments are the only instruments available with an automatic buoyancy correction software feature [8].
A Pfeiffer Thermostar online Mass Spectrometer with 200 amu mass range was used for the detection of selected syngas components. The MS was connected to the gas outlet port of the HP-TGA instruments and operated under EI 70 ionization conditions in the multiple ion detection mode analyzing the main gas components including CH4, Water, CO2 and Carbon Monoxide.
Results and Discussions
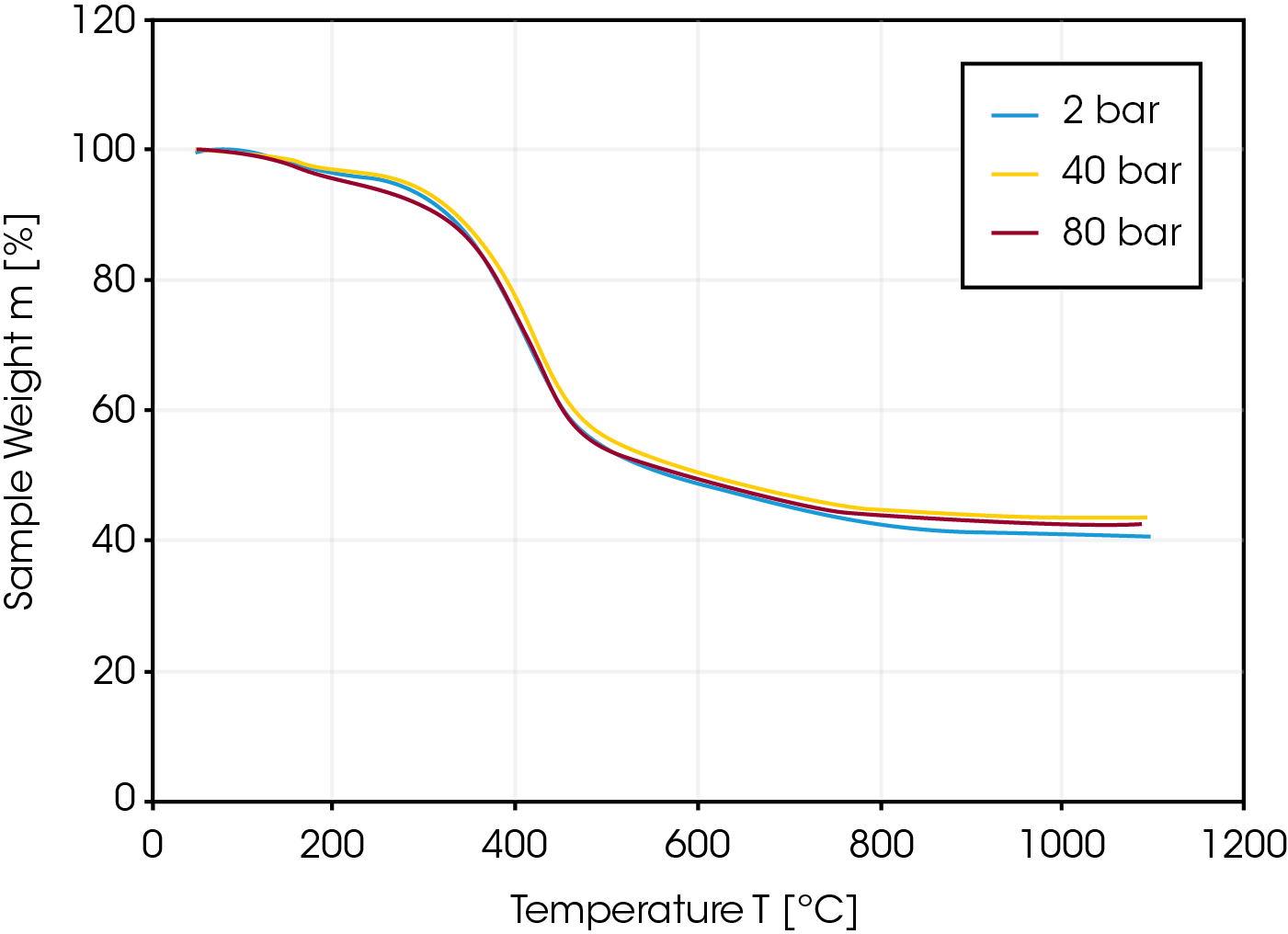
In a first experiment, the pyrolysis of lignite coal was measured at different pressures of 2, 40 and 80 bar to study the influence of pressure on the pyrolysis step of a gasification process. As shown in Figure 3, the kinetics of the pyrolysis step of the lignite is independent of the applied pressure. Not unusual for a lignite sample, the residual weight after pyrolysis is only 40% indicating a high content of humidity and volatile matter.
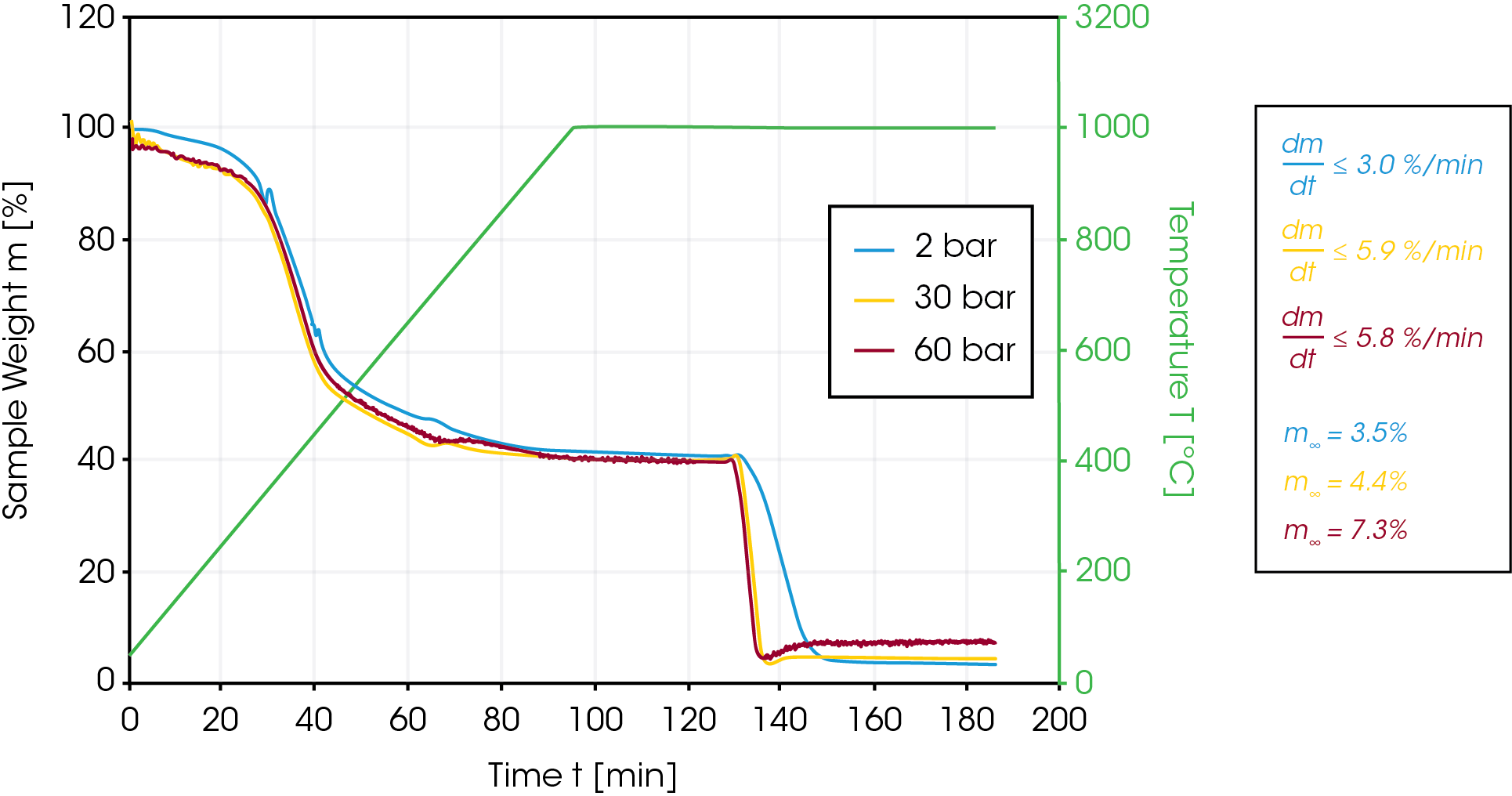
In the next set of experiments, 30% of CO2 were added to the inert argon atmosphere when a constant weight value was obtained after the initial pyrolysis step at 1000 °C. In this case, the pressure was set to 2, 30 and 60 bar. As expected, the reaction rate at 30 bar and 60 bar is significantly higher (5.9 vs 3.0 %/min) compared to the ambient pressure experiment [Figure 4].
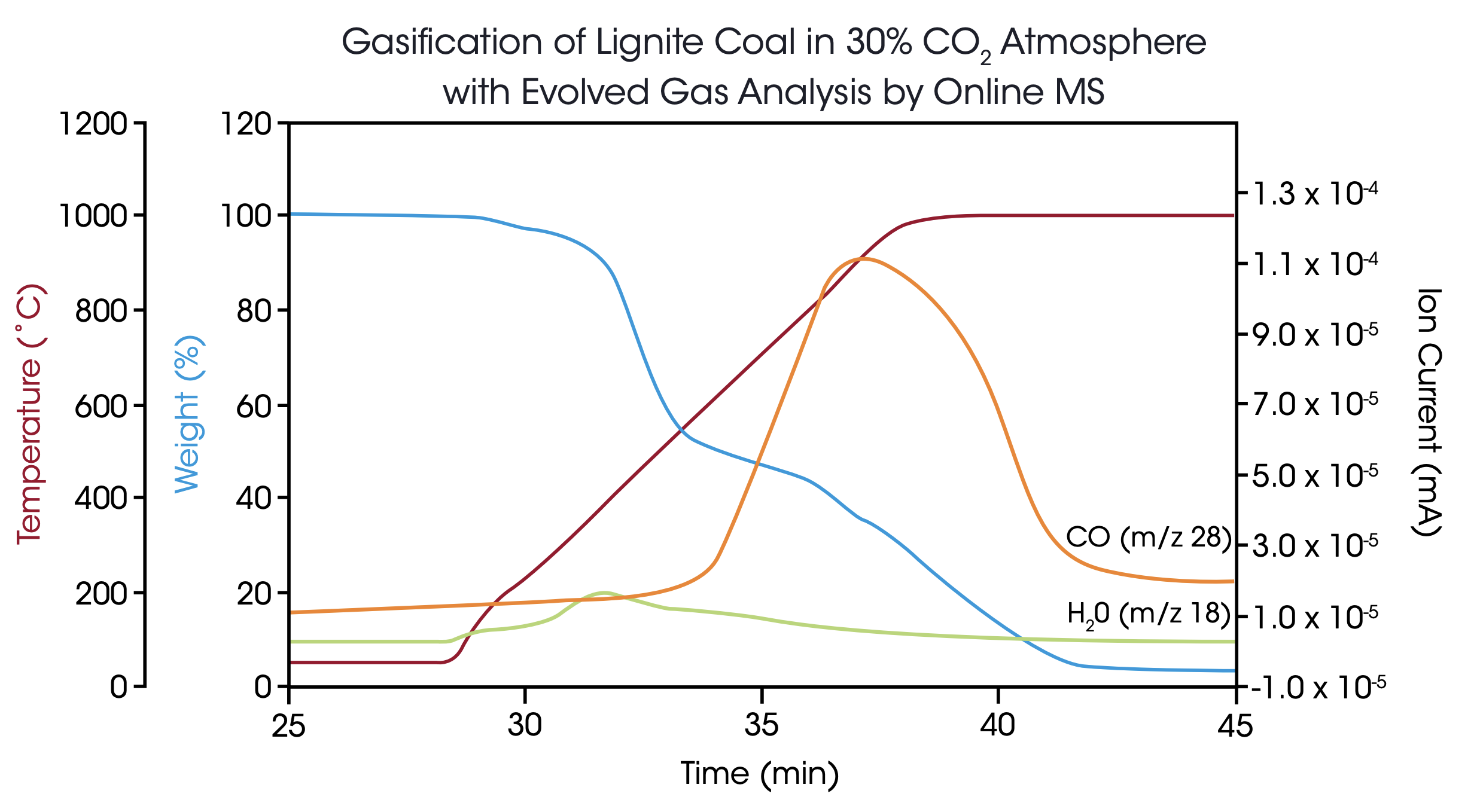
Additionally, the resulting product gas was analyzed by online mass spectrometry to identify the main components and their emission profiles with time and temperature. Figure 5 shows a typical experiment, in this case the lignite sample was exposed to the 30% CO2 atmosphere immediately from the start at 50 °C. At the beginning, H20 was detected in the MS with a maximum at 400 °C. At higher temperatures, carbon monoxide was formed and detected by the m/z trace 28 with a maximum concentration at 900 °C. When the carbon content of the lignite material was completely consumed, the 28 amu signal returned to the baseline value while the final weight signal indicated an ash content of 3.5%.
Four different petroleum coke samples were obtained from a commercial crude oil refinery. As the residual of the refinery process, petroleum coke has a very high carbon and very low volatile matter content and is expected to react considerably slower in gasification processes compared with lignite. Of the ground material, 20 mg samples were used to ensure sample homogeneity. The samples were pyrolyzed at 40 bar and 1100 °C in Argon atmosphere using a heating rate of 100 K/min. After a 30 min isothermal step to ensure constant weight conditions, 30% CO2 were introduced as gasifying agent to start the reaction.
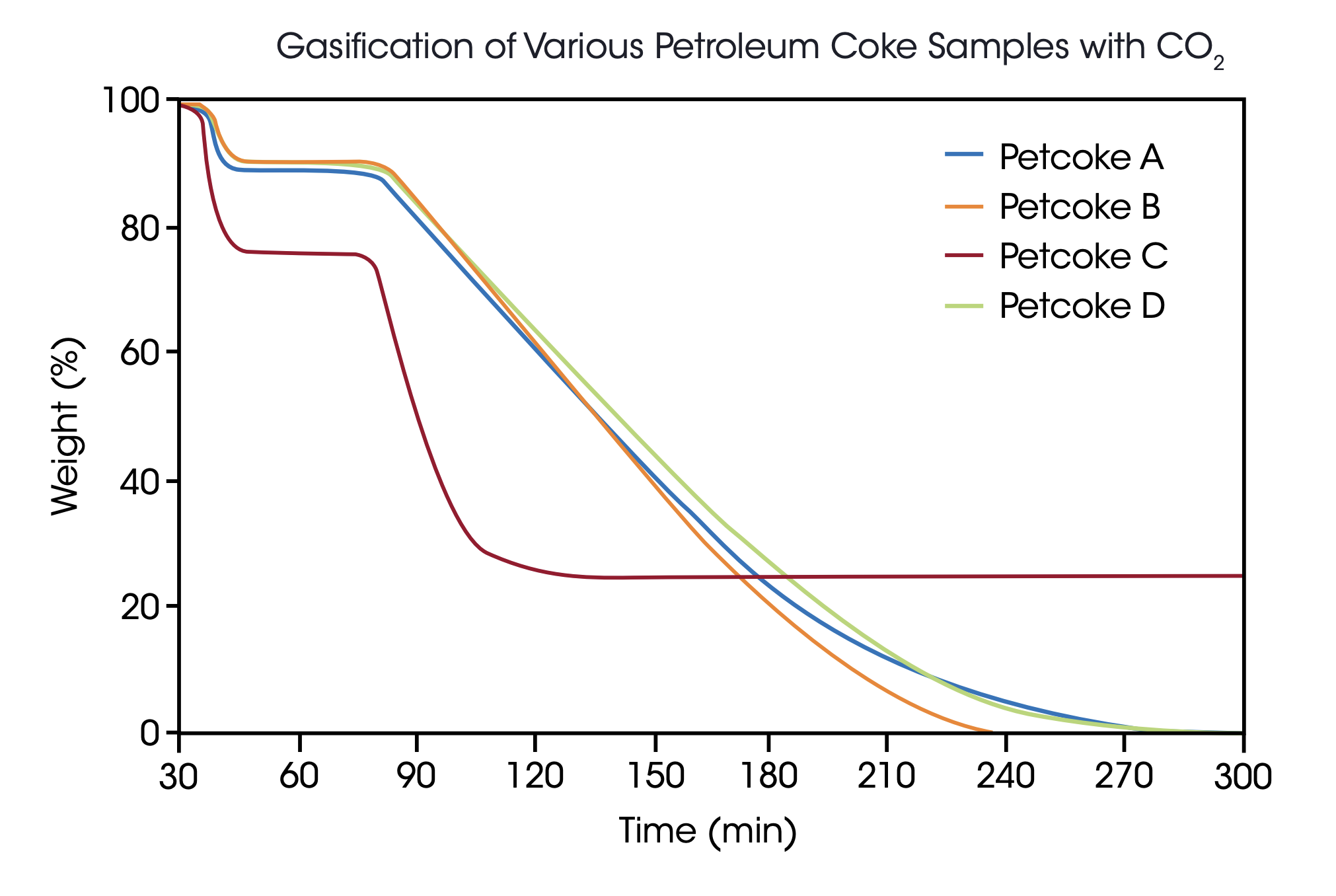
Under these conditions, four hours of reaction time were required to obtain complete conversion for all samples. Three samples showed quite similar properties in the gasification process with 9-10% weight loss in the pyrolysis step and less than 1% of residual ash content while sample C proved to be quite different with 24%content of volatile matter and 25% residual ash. To demonstrate the reliability of these measurements, all samples were run twice and the repeatability obtained was quite acceptable [Table 1].
As a result, it can be concluded that material C, although reacting faster than the other tested materials, will provide a considerably lower syngas yield since the weight loss in the gasification process is only 51% compared with the 89%, 91% and 90% obtained with samples A, B and D.
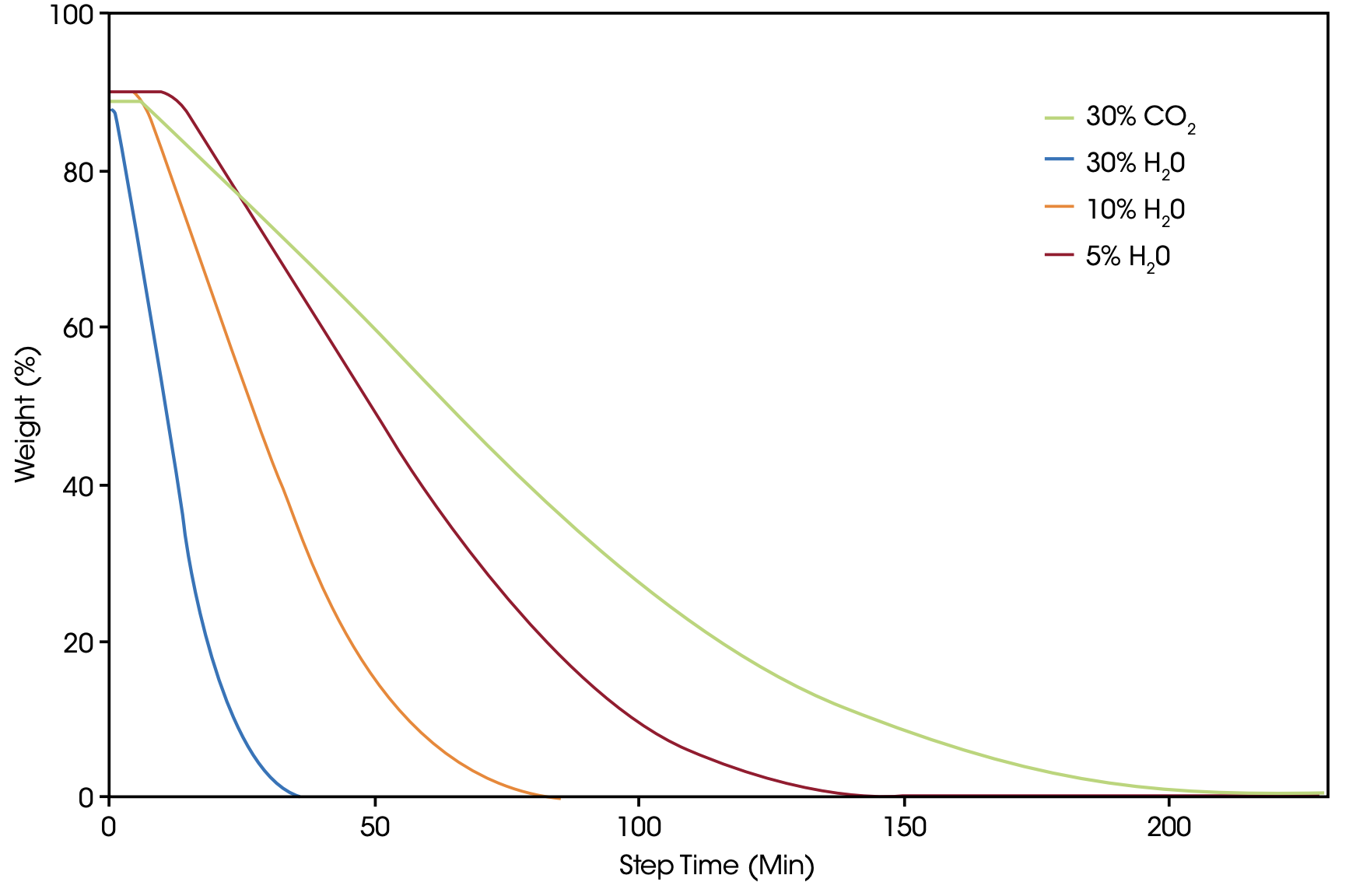
In another set of experiments, petroleum coke sample A was measured under identical conditions with various concentrations of steam in a Discovery HP-TGA 7500 instrument. As expected, steam as gasification agent exhibits the higher reactivity compared with CO2 so that with the highest steam concentration of 30%, the fastest reaction kinetics is obtained. But even the lowest concentration of 5% steam provides a faster gasification reaction than 30% CO2 [Figure 7].
Table 1. Results of replicate measurements of petroleum coke samples A and C.
| Sample | Weight loss Pyrolysis / % | Weight loss Gasificatio / % | Residual ash % |
|---|---|---|---|
| A1 | 10.52 | 88.53 | 1.00 |
| A2 | 10.31 | 88.72 | 0.45 |
| av. A | 10.42 | 88.63 | 0.73 |
| C1 | 24.29 | 50.97 | 25.17 |
| C2 | 24.16 | 50.56 | 25.31 |
| av. C | 24.23 | 50.77 | 25.24 |
Conclusions
The new Discovery HP-TGA product line is well suited for analysis of gasification reactions of organic materials at temperatures up to 1100 °C and pressures in the range from ambient to 80 bar. Measurements performed with Lignite coal and petroleum coke documented that changing the concentration of the applied gasifying agent, here CO2 or steam, directly leads to different process kinetics. The TGA data indicate the reaction yield and also characterize the feedstock regarding the content of humidity, volatile matter and ash. Even more important: the amount of material available for the generation of syngas is measured as well.
The combination with an online Mass Spectrometer proves to be quite useful to measure the changing composition of the generated product gas mixture in correlation with the furnace temperature and the TGA weight signal.
In this study, it could be confirmed that High Pressure TGA is a suitable method to test various gasification feedstock materials under different operating conditions to obtain critical data for the process optimization of gasification plants.
References
- Aranda G., Grootjes A.J., van der Meijden C.M., van der Drift A., Gupta D.F., Sonde R.R., Poojari S., Mitra C.B., “Conversion of High-Ash Coal under Steam and CO2 Gasification Conditions”, Fuel Processing Technology 2016, 141: 16-30
- Pacioni T.R., Soares D., Di Domenico M., Rosa M.F., de F. P.M. Moreira R., José H.J., “Bio-Syngas Production from Agro-Industrial Biomass Residues by Steam Gasification”, Waste Management 2016, 58: 221-229
- Schulze S., Nikrityuk P., Abosteif Z., Guhl S., Richter A., Meyer B., “Heat and Mass Transfer within Thermogravimetric Analyser: From Simulation to Improved Estimation of Kinetic Data for Char Gasification”, Fuel 2017, 187: 338-348
- Buczynski R., Czerski G., Zubek K., Weber R., Grzywacz P., “Evaluation of Carbon Dioxide Gasification Kinetics”, Fuel 2018, 228: 50-61
- da Silva J.C.G., Andersen S.L.F, de F. P. M. Moreira R. JoséH. J., “A Comprehensive Study on By-Products of Food Processing Industry Pyrolysis using a Thermobalance Reactor coupled to GC-FID/TCD: Mass, Atomic and Energy Balances, Thermokinetic Modeling, Product Distribution and Characterization” Journal of Analytical and Applied Pyrolysis 2021, 156: 105107
- TA Instruments: High Pressure Thermogravimetric Analyzers, Product Brochure, 2021
- Dreisbach F., Paschke T., Will C., “New High Pressure TGA Instrument for Coal Pyrolysis and Gasification Measurement under Application Relevant Conditions”, 9th International Freiberg Conference 2018, Berlin
- Paschke T., Dreisbach F., Will C., „A New High Pressure TGA instrument based on the Magnetic Suspension Balance Technology”, 45th Annual Conference of NATAS 2018, Philadelphia
Acknowledgement
This paper was written by Dr. Thomas Paschke, Product Specialist at TA Instruments.
Click here to download the printable version of this application note.

